
¶ Introduction
Specific setups are completed and maintained by business in primarily the LIMS module in the D365FO environment. These setups are based on the specific and unique requirements of the business and is utilized by the various processes and reports available in the LIMS module.
The specific setups are typically completed by senior staff members with special access. These senior users will also be responsible for updating setup as needed on behalf of the laboratory.
Some standard D365 setups required prior to configuring Clinical LIMS setup include:
¶ Navigation
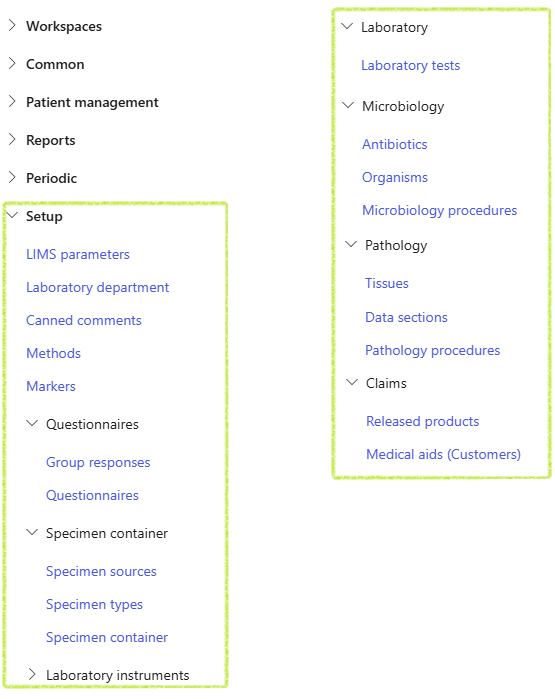
¶ Specific setups
¶ Step 1: Warehouses
Ordering and performing laboratories must be created as Warehouses in standard D365. The warehouse however has to be indicated as a Laboratory so that it may be selected in the LIMS module
- Go to: Warehouse management > Setup > Warehouse > Warehouses
- Select the warehouse record to be defined as a laboratory
- In the General fast tab, set the Laboratory slider button to Yes

¶ Step 2: Users
To allow laboratory users access to process laboratory samples and define levels of access for entering results
- Go to: System administration > Users > Users
- Select the laboratory user from the list
- In the User details fast tab, ensure a Person has been selected for the user
- Set the Laboratory user slider button to Yes

- Expand the Laboratory access fast tab
- To Copy access from (another) User, select the existing user from the dropdown list and click on the Ok button
- Set the relevant slider buttons to Yes to allow the user access to:
- Confidential results (entry and view)
- Verify; Authorize and Edit verified/authorized Laboratory results
- Verify; Authorize and Edit verified/authorized Microbioloy results
- Verify; Authorize and Edit verified/authorized Pathology results
- Add Pathology enter findings data sections that a user must to be restricted to during entry/editing of histology and cytology results. Click on the Add button then select the relevant data section from the dropdown list
- Specify the laboratories the user should have access to for entering results and processing samples, by clicking on the Add button and selecting the laboratory from the dropdown list
A user may have access to various laboratories. The laboratory entered in line 1, will be the user's default laboratory when they sign in to D365. It is therefore suggested to ensure the main laboratory where the user is stationed should be entered in line 1. To make the list user friendly, enter the additional laboratories in alphabetic order. Use the Add and Remove buttons to move the laboratories in the correct position.
- If a user should be restricted to enter or manage results for specific departments only for a selected laboratory, then select the Department from the dropdown list in the Enter results department restrictions grid.
If the user should have the same department restrictions for all laboratories, set the restrictions for one laboratory, then use the Copy from laboratory function to copy the access for other laboratories the user has access to.

¶ LIMS parameters
¶ Step 3: General parameters
- Go to: Clinical lab information management > Setup > LIMS parameters
- Open the General tab
- Expand the Bar codes Fast tab
- Select the appropriate Barcode setup from the dropdown list if required
- Enable the SSRS label printing if required
- Select the appropriate Default label layout data source ID from the dropdown list
- Select the appropriate Primary container default label layout ID from the dropdown list
- Select the appropriate Aliquot container default label layout ID from the dropdown list
- Select the appropriate Document label label layout data source ID from the dropdown list
- Select the appropriate Doument label layout ID from the dropdown list
The label layouts have to be created in the Warehouse management module prior to the parameter selection in LIMS. These default layouts will automatically be applied when barcodes as printed in the Clinical LIMS module for specimen containers.
- Expand the Azure fast tab and select the appropriate Azure storage container for test reports from the dropdown list if required

- Expand the Samples fast tab
- To customise sample statuses, set the Customize sample statuses slider button to Yes. Enter the alternate status description to use for each standard status.
It is advised to utilise the standard Status descriptions. Should a company however require alternate naming for the sample statuses, it is advised to set this before production implementation as a once-off setup by the consultant. Once set, these values should not be changed as it may affect the integrity of existing samples.
- Expand the Laboratory results fast tab
- Enter a Print number format to be utilized in Clinical LIMS forms, related to the setup of Laboratory tests; Microbiology procedures and Pathology procedures.
- In the Lab result flags grid, enter an Identifier for each indicated abnormal result type. If required, select the colour application; colour application type; colour and font type to be applied for each abnormal result type listed.
The identifiers; colour selection and font types will be used to indicate the abnormal and critical results in the various LIMS forms and reports.
- In the Report trailers memo boxes for Lab (Clinical laboratory), Mic (Microbiology) and Pth (Anatomical pathology i.e. Histology and Cytology), add contact details which providers may use to contact the laboratory for consultation in regards to the results.
- Expand the Patients fast tab
- Select the Default identification type from the dropdown list to be used in new laboratory visit requests.
- Enter the Default name for new borns to be used in new laboratory visit requests.

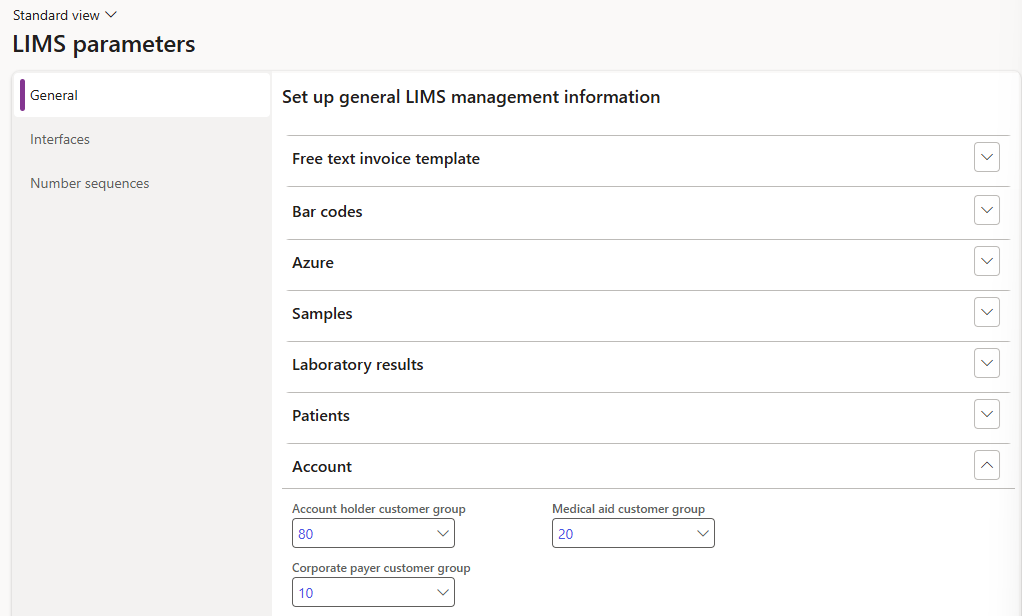
- Expand the Account fast tab
- Select the Account holder customer group from the dropdown list
- Select the Medical aid customer group from the dropdown list
- Select the Corporate payer customer group from the dropdown list
The customer groups selected, will automatically default when customers are created as LIMS account holders to ensure accurate selections during this process.
- Select the appropriate Default visit diagnostic codes (ICD10 Codes) for Lab, Mic and Pth test orders. These codes will default for claims purposes when there were not diagnosis specific codes specified for the visit.
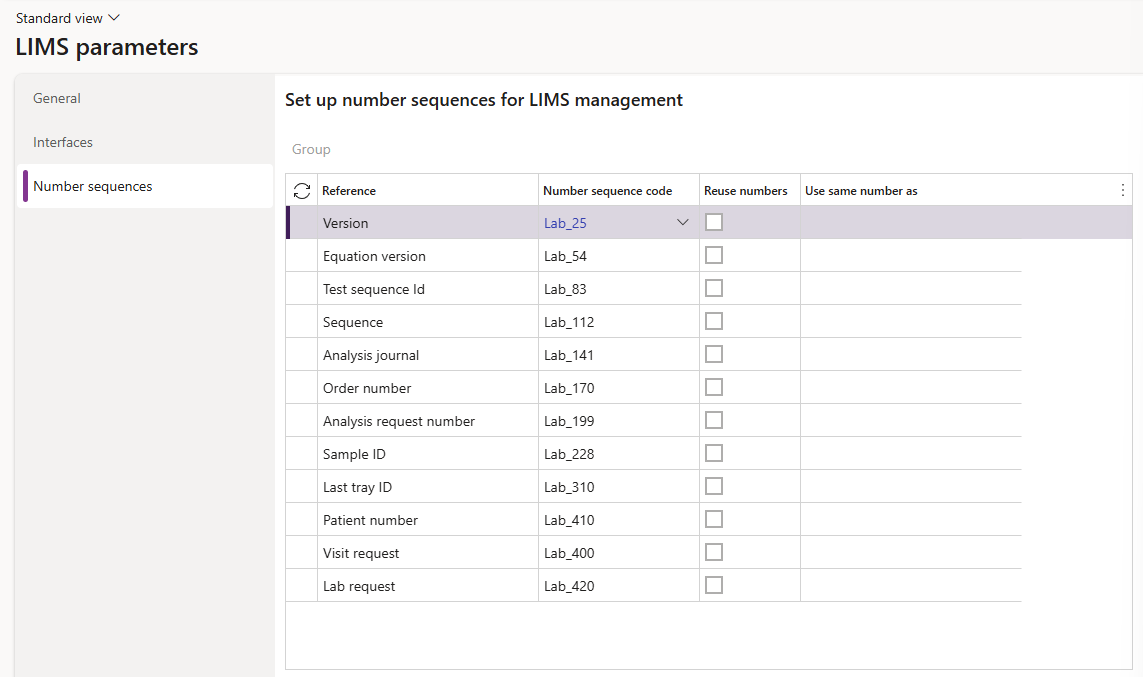
¶ Step 4: Number sequences
- Go to: Clinical lab information management > LIMS parameters > Number sequences
- Select the appropriate Number sequence codes for the various numbers for LIMS where required, including:
- Version
- Sample ID
- Patient number
- Visit request
- Lab request
¶ LIMS General clinical laboratory setups
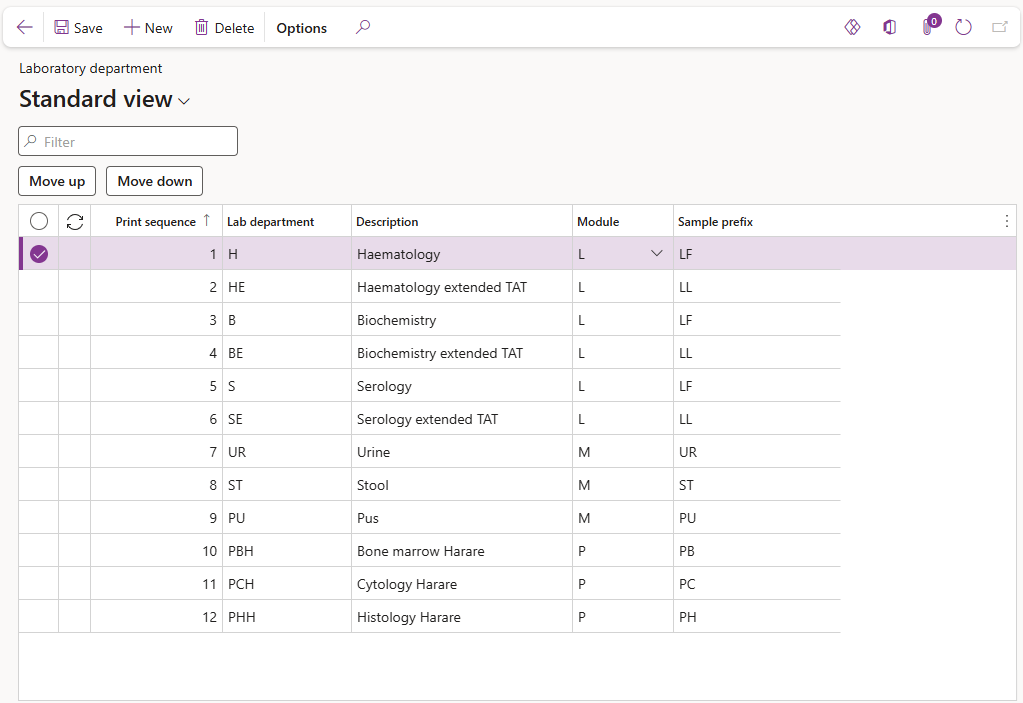
¶ Step 5: Laboratory departments
Laboratory departments may be set up for individual departments; benches; methods or other required definitions to identify specific analysis areas within laboratories. These departments may be used to restrict workers, to ensure they only enter, verify and authorize analysis results for which they are qualified.
Laboratory departments are used to group together tests and procedures related to laboratory disciplines and may be used in various forms to filter samples and results based on the department and for statistics.
Sample prefixes are specified per department to allow tests from various departments, for the same laboratory module and same prefix, to be grouped together in a single sample for resulting and reporting purposes.
The print sequence for laboratory reports is also selected per department within this setup form.
- Go to: Clinical lab information management > Setup > Laboratory department
- Click on the New button in the Action pane
- Enter a short Lab department name
- Enter a longer lab department Description
- Select the Module from the dropdown list i.e. L for Laboratory; M for Microbiology or P for Anatomical pathology.
- Select a short Sample prefix
The sample prefix will be utilised in Sample number creation and will allow tests that should be reported together, to be included in the same sample for resulting and reporting purposes.
- Use the Move up or Move down buttons to move the department to the correct print position. The print sequence number will automatically be updated and entered as departments are added; deleted or moved into position.
The laboratory department print sequence allows results to be sorted in the correct sequence in LIMS forms in D365 and on laboratory reports based on the department selected for the test or procedure.
A department may only be deleted if it is not already used in another LIMS form e.g. laboratory test. An error message will appear indicating this is now allowed if attempted.

¶ Step 6: Canned comments
Pre-defined comments used regularly by the laboratory, may be captured in the Canned comments setup form. These comments may be inserted in various forms in the LIMS module including new Laboratory visit requests; when entering results and automatically defaulted for specific tests etc. Additionally these comments also allow default selections to be added for comments used during the entering of findings for Anatomical pathology samples.
- Go to: Clinical lab information management > Setup > Canned comment
- Enter data in the Filter field to find a specific record or click on the record card in the filter pane of the required record to view or edit the record. The card details include the name and description of the canned comment as well as the module selected for the record if any.
The filter pane displays active records only. To list active and inactive records; set the Show inactive slider button to Yes.
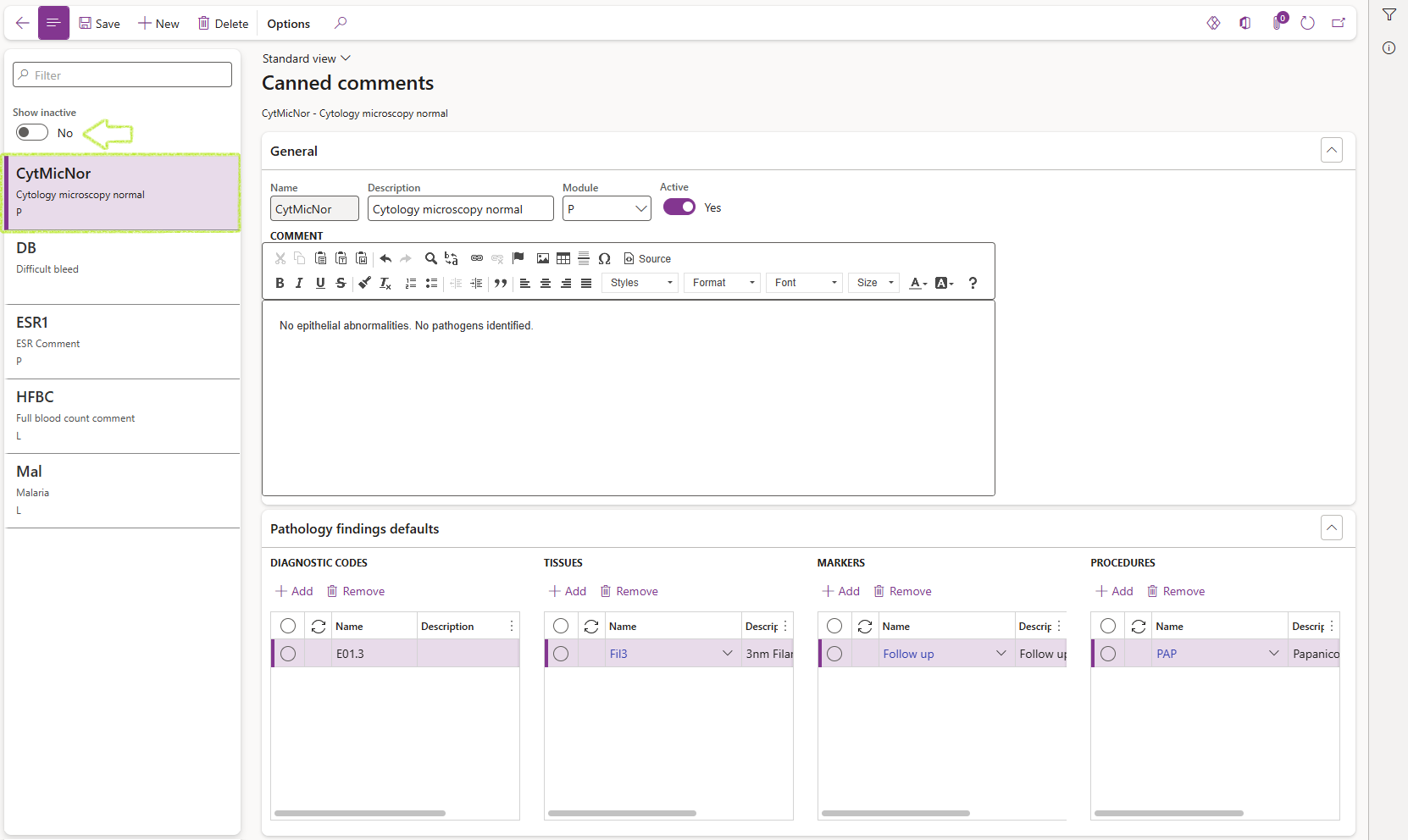
- To create a new Canned comment, click on New in the Action pane
- Complete the required fields in the General fast tab:
- Enter a short Name (maximum 15 characters)
- Enter a longer Description of the canned comment to indicate its purpose or content
- Select a Module from the dropdown list if use of the canned comment must be restricted to a specific module.
- Enter the text in the Comment memo box which will be displayed wherever this comment is selected in D365 LIMS.
- Complete the optional Pathology findings fast tab if required
- Diagnostic codes e.g. ICD10 Codes
- Tissues
- Markers
- Procedures
- Click on the Add button for the required grid in the Pathology default selections fast tab.
- Select the required default option form the dropdown menu.
- Once all required fields have been completed; set the Active slider button in the General fast tab to Yes to make the canned comment available for use.
- Click on the Save button in the Action pane if required. D365 does however automatically save the entries to the form.
- Go to: Clinical lab information management > Setup > Methods
- Enter data in the Filter field to find a specific record or click on the record in the list to view or edit the record.
- To create a new Method, click on New in the Action pane
- Enter a short name for the Method
- Enter a longer Description
- Click on the Save button in the Action pane if required. D365 does however automatically save the entries to the form.
If the Module has been selected as P (Anatomical pathology) in the General fast tab, the Pathology findings defaults fast tab and grids will be available for selecton. Default selections may be added, which will then automatically be added to Anatomical pathology sample when the canned comment is selected during the entering of pathology findings. The default selection grids include:
To add default selections for Pathology samples:
A canned comment record cannot be deleted or be made inactive if it is referenced by other active records. Refer to the related information pane fact boxes to view which recorrds the selected canned text may be referenced by.
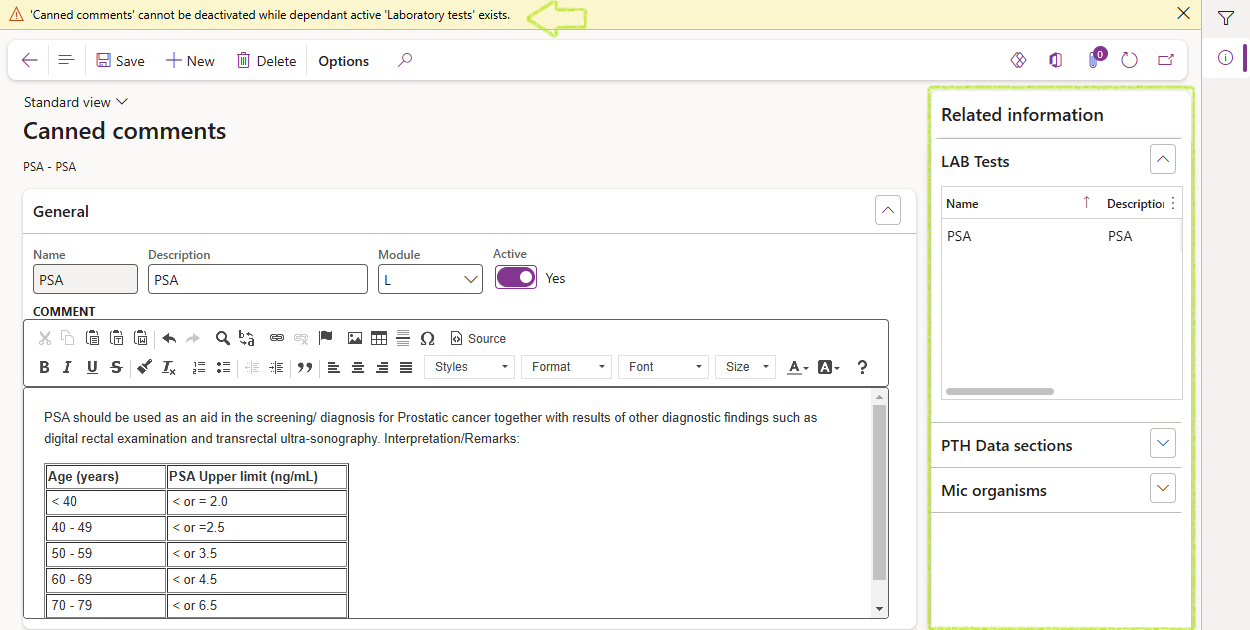
¶ Step 7: Methods
Methods are used in various LIMS setup forms to define the method used during testing. Methods are typically created for the actual analysis method or the instrument used.
The list displays active records only by default. To list active and inactive records; set the Show inactive slider button to Yes.
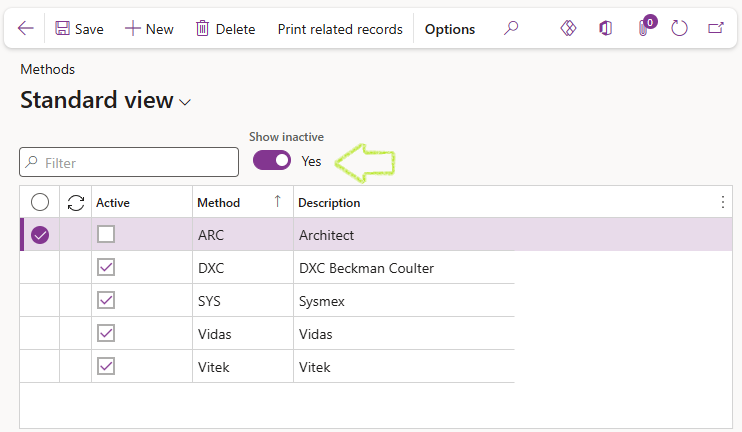
A Method cannot be deleted and should not be made inactive if it is referenced by other active records. Error messages will appear should this be attempted.
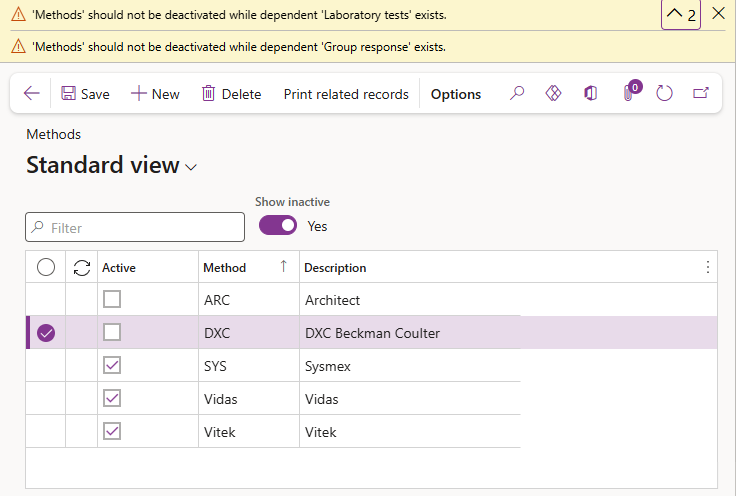
To compile a list of records in which the current method is referenced:
- Click on Print related records in the Aciont pane.
- The method, selected in the list, will default to the Records related to Methods dialogue parameters that appears. Select another method from the dropdown if required or clear the method field to compile a list of related items for all methods.
- Select what other records to include in the list:
- Select Yes or No to check for an include Group responses records the selected method may be referenced in.
- Select Yes or No to check for an include Lab tests records the selected method may be referenced in.
- Select Yes or No to check for an include Mic procedures records the selected method may be referenced in.
- Select Yes or No to check for an include Instruments records the selected method may be referenced in.
- Select Yes or No to Include inactive records the selected method may be referenced in.
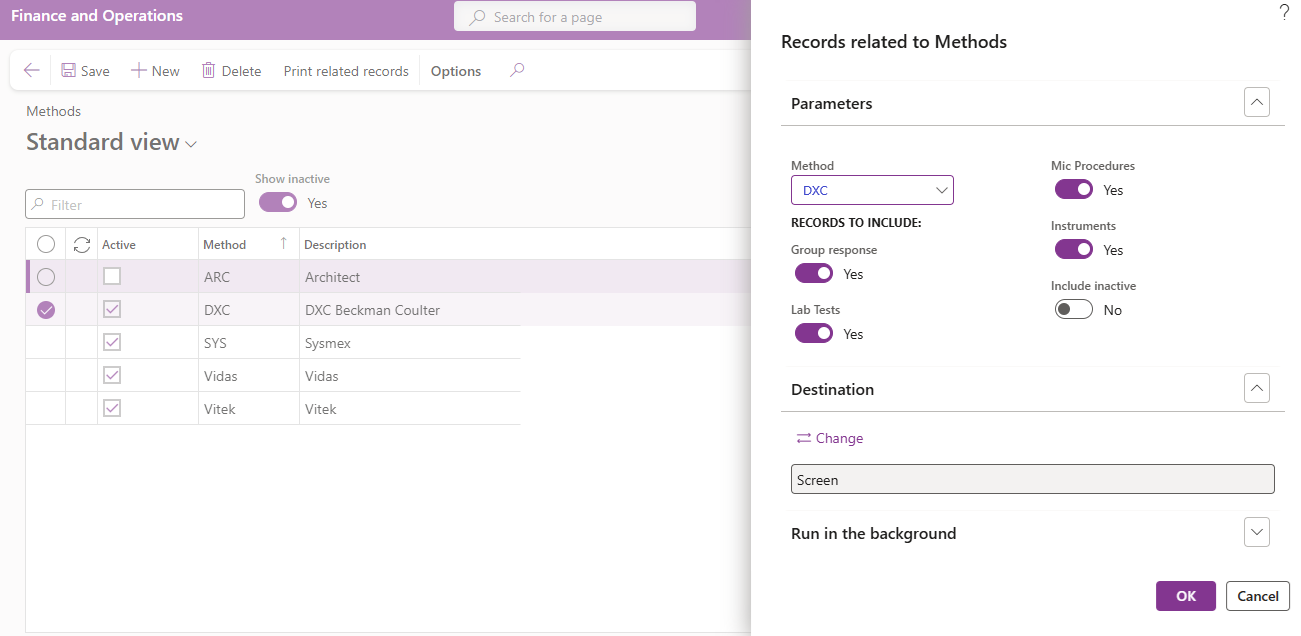
- Click on the OK button to compile the report.
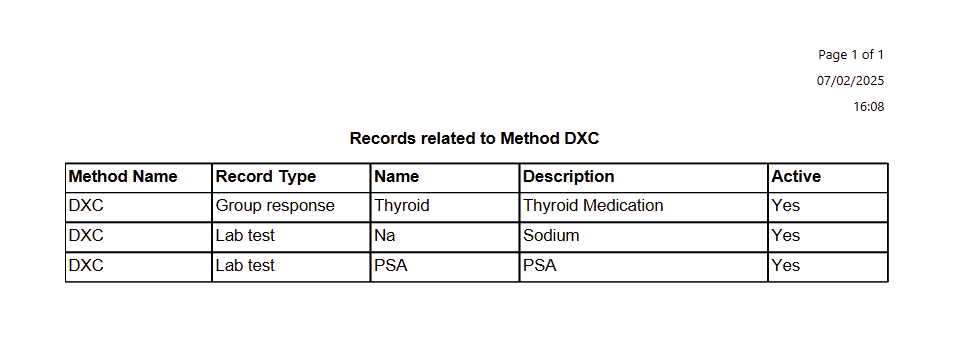
The report lists the related Method; the record type it is included in as well as the related record name; description and if that record is active or not.
¶ Step 8: Markers
Markers may be used to flag patients or patient visits so that the patient or visits may be filtered using these markers. An example of a marker may be when a patient tests positive for Malaria and this should be considered in any subsequent tests for malaria for the patient.
- Go to: Clinical lab information management > Setup > Markers
- Enter data in the Filter field to find a specific record or click on the record in the list to edit the record.
- Click on the Show inactive slider button to list both active and inactive marker records.
- To create a new Marker, click on New in the Action pane
- Enter a short name for the Marker
- Enter a longer Description
- Select the applicable Module where relavant from the dropdown list, else leave the field empty when the marker may be used in all modules
- Select Save to patient if the marker should be saved to the patient file, as opposed to only for specific visits.
- Click on the Save button in the Action pane if required. D365 does however automatically save the entries to the form.
- If a Marker is no longer is use but has been actively used for patients and visits; it is suggested to rather inactivate the record than delete it. To inactive a record, click on the Active checkbox for the relevant record.

A Marker cannot be inactivated or deleted whilst it has a dependent record. Expand the related information pane to view the various records in which this Marker may have been included in order to remove it from the dependant record first.
¶ Step 9: Questionnaires
Questionnaires may be used during the ordering or resulting of tests and allows the client to create a unique list of questions and answers for users to complete.
¶ Step 9.1: Create a Group response
Group responses are a list of possible answers a user may selected for a question asked within a questionnaire.
- Go to: Clinical lab information management > Setup > Questionnaires > Group responses
- Enter data in the Filter field to find a specific record or click on the record in the list to edit or view the record. Click on the Show inactive slider button to list either Active or both Active and Inactive records.
- To create a new Group response:
- Click on New in the Action pane
- Enter a short Name for the record in the General fast tab
- Enter a longer Description
- Select the appropriate Type from the dropdown list i.e. Order or Result which indicates the purpose of the group response record and in which type of questionnaires it may be selected
- If the group response may only be used for a specific module, select the approriate Module from the dropdown, else leave the field empty.
- The Display will default to Description, however this may be changed to Identifier by selecting it from the dropdown list. This indicates if the Description or Identifier must be displayed on reports.
- For a Results type Group response, select a Default method from the dropdown list if required
- Expand the Responses fast tab
- Click on the Add fast tab button to add a new response line
- Enter a short Identifier
- Enter a longer Description
- Repeat the process of adding response lines until all required responses are listed
- Additional selections and entries can be defined for Results type Group responses for Microbiology and Pathology. These entries and selections will automatically be applied in the enter results/findings forms:
- If a specific result should default, select the response line then click on the Set default fast tab button.
- If a result should be flagged as abnormal, select the Abnormal result check box in the grid for the appropriate response line
- If a marker should automatically default , select the appropriate Marker in the dropdown list in the grid
- For Pathology only, if follow-up is required by the laboratory, enter a number in the Pth Follow up days column
- For Microbiology, select Mic Inf Ctrl if the result should be flagged for infection control
- For Microbiology, select a Mic method from the dropdown list, to display the method for the result if required
- For Microbiology, enter Mic delta hours to indicate how many days of previous results to consider to perform a delta check if required
- Click on the Active slider button in the General index tab, to make the record active and allow for selection in a Questionnaire.
- Click Save in the Action pane to save the record

- If a Group response is no longer is use but has been actively used in other records; it is suggested to rather inactivate the record than delete it. To inactive a record, click on the Active slider button in the General fast tab.
A Group response cannot be inactivated or deleted whilst it has a dependent record. Expand the related information pane to view the various records in which this Group response may have been included in order to remove it from the dependant record first.
¶ Step 9.2: Create a Questionnaire
A Questionnaire is a list of questions which may be asked during the ordering of tests or as a way to enter results for Microbiology or Pathology. Questionnaires may include questions with different types of answers including Group responses (see previous steps). Conditions may be set to allow some questions to be asked or skipped or even to be made required when the conditions are met.
- Go to: Clinical lab information management > Setup > Questionnaires > Questionnaires
- Enter data in the Filter field to find a specific record or click on the record in the list to edit or view the record. Click on the Show inactive slider button to list either Active or both Active and Inactive records.
- To create a new Questionnaire:
- Click on New in the Action pane
- Enter a short Name for the record in the General fast tab
- Enter a longer Description
- Select the appropriate Type from the dropdown list i.e. Order or Results
If the Questionnaire type is set to Order, then it can only be selected as an Order screen for a test or procedure which will then be completed during the ordering of tests for a visit.
If the Questionnaire type is set to Results, then it can only be selected for use during the entry of Microbiology or Pathology results.
- If the group response may only be used for a specific module, select the approriate Module from the dropdown, else leave the field empty so it may be used in all modules
The Active slider button will default to No when the record is created. The records can only be made active when at least one question has been added and all required fields have been completed.
- Expand the Questions fast tab
- Click on the Add fast tab button to add a question line
The line number will automatically default to the next available number in numeric sequence when a line is added. Quwstions may be reordered by clicking on the Up or Down fast tab buttons. When a line is removed, the line numbers will also automatically update if affected by the modification.
- Enter a short Question ID
- Enter the Question as it will be displayed to the user when it is required to be answered
- Select the Answer type from the dropdown list which will determine the way the question is answered. The following answer types are available:
- Yes/No answer: This answer type will display to the user as a Yes/No slider button
- Group response: An existing Group response can be selected of which the responses will then appear as a dropdown list
- Free text: This answer type is a short text field used for entering shorter text answers e.g. a name
- Paragraph: This answer type allows for entry of longer free text answers in a paragraph format
- Number: This answer allows the entry of a numeric value only
- Date: This answer allows the selection of a date from a calender or manual entry of data in a date format
- If the answer type was selected as a Group response, then select the appropriate Group response from the dropdown list. The column will not be available for selection for any other answer types
Only Group responses that are Active and match the Type of the Questionnaire i.e. Results or Order as well as the Module i.e. L; M; P; G or no module, which is allowed for use with all other modules, will be available in the dropdown list for selection.
- Select the appropriate Answer option from the dropdown list:
- Required: The answer is mandatory and cannot be skipped
- Optional: The answer is not mandatory and may be skipped or answered
- Conditional: Conditions may be set for when the question should be asked or skipped or made mandatory
- If the question and answer should appear on the laboratory report to the provider, select the Reportable check box

- If the Answer type for a question was selected as Conditional, then the Conditions queries are enabled below the grid to allow the setting of conditions based on the answer of another previous question, in order to determine what action to be taken.
For example: If a patient has not been vaccinated for COVID-19 before, you would not need to ask when they were vaccinated. Therefore the condition would be, if they have not been vaccinated before to skip the question regarding the vaccination date.
- Complete the Conditions fields as follow:
- Select the Question ID from the dropdown list, of the question of which the answer will determine the action for the current question
- Select the approporiate Criteria from the dropdown list
- Enter the Answer that should be evaulated to meet this condition
- Select the appropriate Action from the dropdown list i.e Ask; Skip or Require

- If the Questionnaire type is Results and the module selected M, then the Result reference ranges field group will be enabled below the Questions grid. This section allows the entry of reference ranges with the correct decimals and units, to be utilised during the entry of Microbiology results and to be displayed on Laboratory reports. If required, complete the fields as follow:
- Enter a number in the Decimals field to indicate with how many decimal places the reference ranges must be displayed.
- Enter a Low value if required
- Enter a High value if required
- The Reference range will default based on the Low and High values entered. The range may however be manually changed to display another value e.g. < 5 instead of 0 - 5
- Select the appropriate Unit from the dropdown list if required

- To ensure the questionnaire has been created correctly, click on Preview in the Action pane.

- The Questionnaire preview dialogue will appear. The dialogue lists the questions and may be used to test the questionnaire to ensure it reacts as expected based on selected Answer options and Conditions. Click on the Close button to exit from the dialogue.
- If necessary, make corrections to the Questionnaire based on testing completed in the Preview dialogue and repeat the process of reviewing via the Preview dialogue until the questionnaire works as expected.
- Once the Questionnaire is ready for use, click on the Active slider button in the General fast tab, to make the Questionnaire available for use in other forms.
- If a Questionnaire is no longer is use but has been actively used in other records; it is suggested to rather inactivate the record than delete it. To inactive a record, click on the Active slider button in the General fast tab.
A Questionnaire cannot be inactivated or deleted whilst it has a dependent record. Expand the related information pane to view the various records in which this Questionnaire may have been included in order to remove it from the dependant record first.
¶ Step 10: Specimen containers
Specimen containers are used to collect samples from patients for analysis. These Specimen containers are recorded in D365 to allow the containers to be specified for Laboratory tests; Microbiology procedures and Pathology procedures which then allows the containers required, to be automatically listed for collection, when the tests and procedures are ordered for a laboratory visit. Specimen sources and Specimen types are also recorded which may then be used to further define the Specimen container and is also used in ordering of Microbiology and Pathology procedures.
¶ Step 10.1: Create Specimen sources
Specimen sources refer to how or from where in the body the specimens were collected e.g. left arm (for blood) or midstream (for urine).
- Go to: Clinical lab information management > Setup > Specimen container > Specimen sources
- Enter data in the Filter field to find a specific record or click on the record in the list to edit or view the record.
- To create a new Specimen source:
- Click on New in the Action pane
- Enter a short name in the Specimen source column
- Enter a longer Description for the specimen source
- Click on Save in the Action pane

- To completely remove a Specimen source from D365, select the record in the grid then click on Delete in the Action pane.
A Specimen source cannot be deleted whilst it has a dependent record. Expand the related information pane to view the various records in which this Specimen source may have been included in order to remove it from the dependant record first.
¶ Step 10.2: Create Specimen types
Specimen types refers to the kind of specimen e.g. blood or urine.
- Go to: Clinical lab information management > Setup > Specimen container > Specimen types
- Enter data in the Filter field to find a specific record or click on the record in the list to edit or view the record.
- To create a new Specimen type:
- Click on New in the Action pane
- Enter a short name in the Specimen type column
- Enter a longer Description for the specimen type
- If the Specimen type is to be used in Microbiology Culture and Susceptibility analysis, and if relevant, select a M.I.C Susceptibility target from the dropdown list to be utilised as the default target during entry of the analysis results.
- If the Specimen type is to be used for Microbiology orders, Specimen sources may be specified to improve the ordering process. To add Specimen sources:
- Click on the Add button for the Specimen sources grid
- Select the relevant Specimen source from the dropdown list. To add more Specimen soures for the specimen type, repeat this step
- To remove a specimen source, select the specimen source in the grid, then click on the Delete button of the Specimen sources grid
- Click on Save in the Action pane

- To completely remove a Specimen type from D365, select the Specimen type in the grid then click on Delete in the Action pane.
A Specimen type cannot be deleted whilst it has a dependent record. Expand the related information pane to view the various records in which this Specimen type may have been included in order to remove it from the dependant record first.
¶ Step 10.3: Create Specimen containers
Before Specimen containers may be recorded in D365, the inventory Item code for the container must first be defined as a Released product.
- Go to: Clinical lab information management > Setup > Specimen container > Specimen container
- Enter data in the Filter field to find a specific record or click on the record in the list to edit or view the record. Click on the Show inactive slider button to list either Active or both Active and Inactive records.
- To create a new Specimen container:
- Click on New in the Action pane
- Enter a short Name for the container
- Enter a longer Description
- Select the Item number from the dropdown list. This is a mandatory field to complete
- Select the Specimen type from the dropdown list if relevant
- Select the Specimen source from the dropdown list if relevant
- Select the Stability in hours
- The Count slider button will default to No, which will then require a standard volume to be defined for the container. If the cliser button is set to Yes, then a Count should be entered instead to indicate how many of the container is typically required e.g. Microbiology containers would typically be measured with a count whilst blood containers would be measured in volume.
- Enter either a standard Volume for the container in millileters or a Count, depending on the setting of the Count slider button
- Click on Save in the Action pane

- If a Specimen container is no longer is use but has been actively used in other form; it is suggested to rather inactivate the record than delete it. To inactive a record, click on the Active slider button in the General fast tab.
- To completely remove a Specimen container from D365, select the Specimen container then click on Delete in the Action pane.
A Specimen container cannot be deleted whilst it has a dependent record. An error message will appear if attempted