¶ Introduction
Companies in process industries usually manufacture products that are subject to varying degrees of regulation by regional, national or global organizations to safeguard workers and/or consumers. For instance, DDT is banned in most countries but allowed in some countries; or use of asbestos is completely banned but use of benzenes up to 5mg/Kg in toys is allowed in EU.
Process Manufacturing for Microsoft Dynamics 365 provides a rich toolset for food, drug and chemical manufacturers to manage products containing restricted and/or regulated substances. Process manufacturing solution has been extended further by several ISV’s to meet specific requirements of chemical and pharmaceutical manufacturers.
Restricted product:
A product is considered to be a restricted product if its distribution is limited or constrained by a government authority.
Regulated product:
A product is considered to be a regulated product if it is regulated by a government authority and if it requires compliance and audit reporting.
Reported product:
Reported product is a product that is regulated and a company is required by regional or national authority to provide usage data on the product.
Product safety data sheet:
Product safety data sheet is a brochure that contains handling, emergency and other related information about the product for example; flash point, boiling point etc. of the substance.
¶ Navigation

¶ Specific setups
¶ Step 1: Setup Restricted product lists
In Dynamics 365 restricted products are managed through established “Restriction lists” for countries and regions. Restricted products are set up in the compliance section of either the Inventory management or the Product information management module.
There are two types of lists – inclusive and exclusive. Inclusive type lists must be set up for a country before exclusive type lists may be set up for its regions.
¶ Step 1.1: Setup Restricted product lists for countries
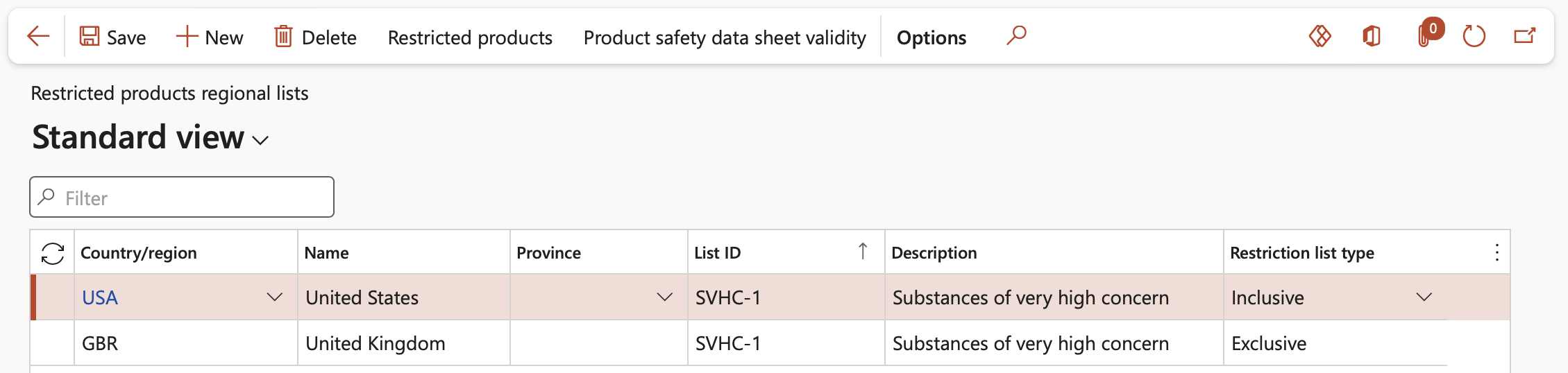
Go to: Product information management > Setup > Product compliance > Restricted products regional lists
- On the Action pane, click the New button
- In the Country/region field, select the country or region for which the list is being created
- In the List ID field, enter a naming convention for this list
- In the Description field, enter a more detailed value to associate with the List ID
- In the Restriction list type field, select whether the list is Inclusive or Exclusive. These list types are as follows:
- Inclusive: Products distributed under the list are not restricted and may be sold in this country (USA). This selection type may only be made at the country level.
- Exclusive: Products distributed under the list are restricted and cannot be sold in any country, state, or province (GBR) in which the list is valid. This selection type may be applied at the country, state, or province level.
- To create a list for another country or region, repeat the steps above
If the number of regions in which product is allowed is greater than the number in which it is not allowed, then set up an inclusion list for the country and an exclusion list for state/provinces where the product is restricted
¶ Step 1.2: Setup Restricted product lists for state/province
Go to: Product information management > Setup > Product compliance > Restricted products regional lists
- On the Action pane, click the New button
- In the Country/region field, select the country or region for which the list is being created
- In the State field, select the relevant State from the dropdown list
- In the List ID field, enter a naming convention for this list
- In the Description field, enter a more detailed value to associate with the List ID
- In the Restriction list type field, select whether the list is Inclusive or Exclusive
- To create a list for another state, repeat the steps above
¶ Step 1.3: Add items to the Restricted product regional lists
- Once the restriction lists are set up, items or products may be added to these lists
- Select the list to add the products to
- On the Action pane click on the Restricted products button
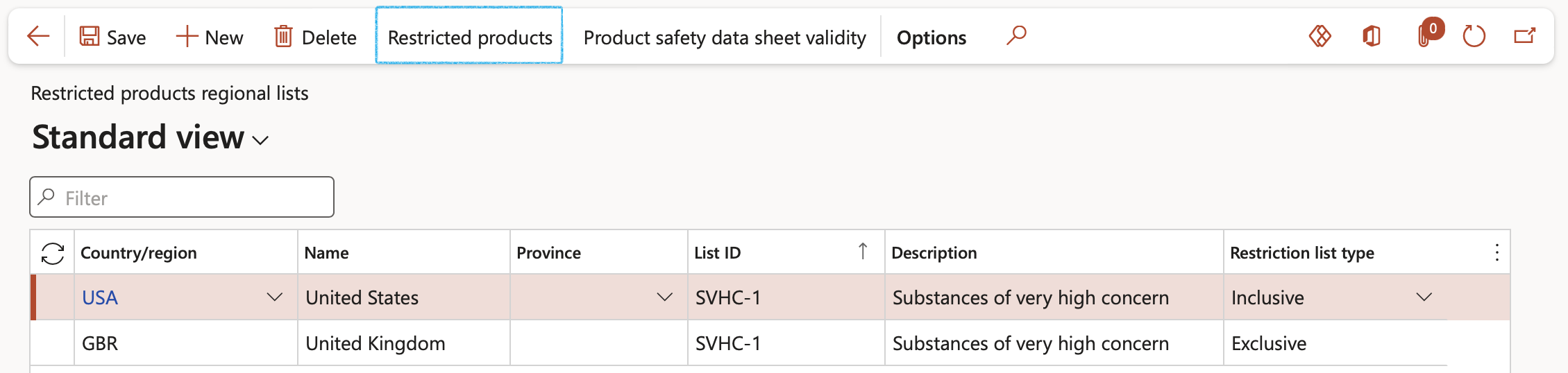
- On the Restricted products form, in the Action pane, click on the New button
- In the Item number field, select the relevant item from the dropdown list.
- The Product name field will automatically be populated
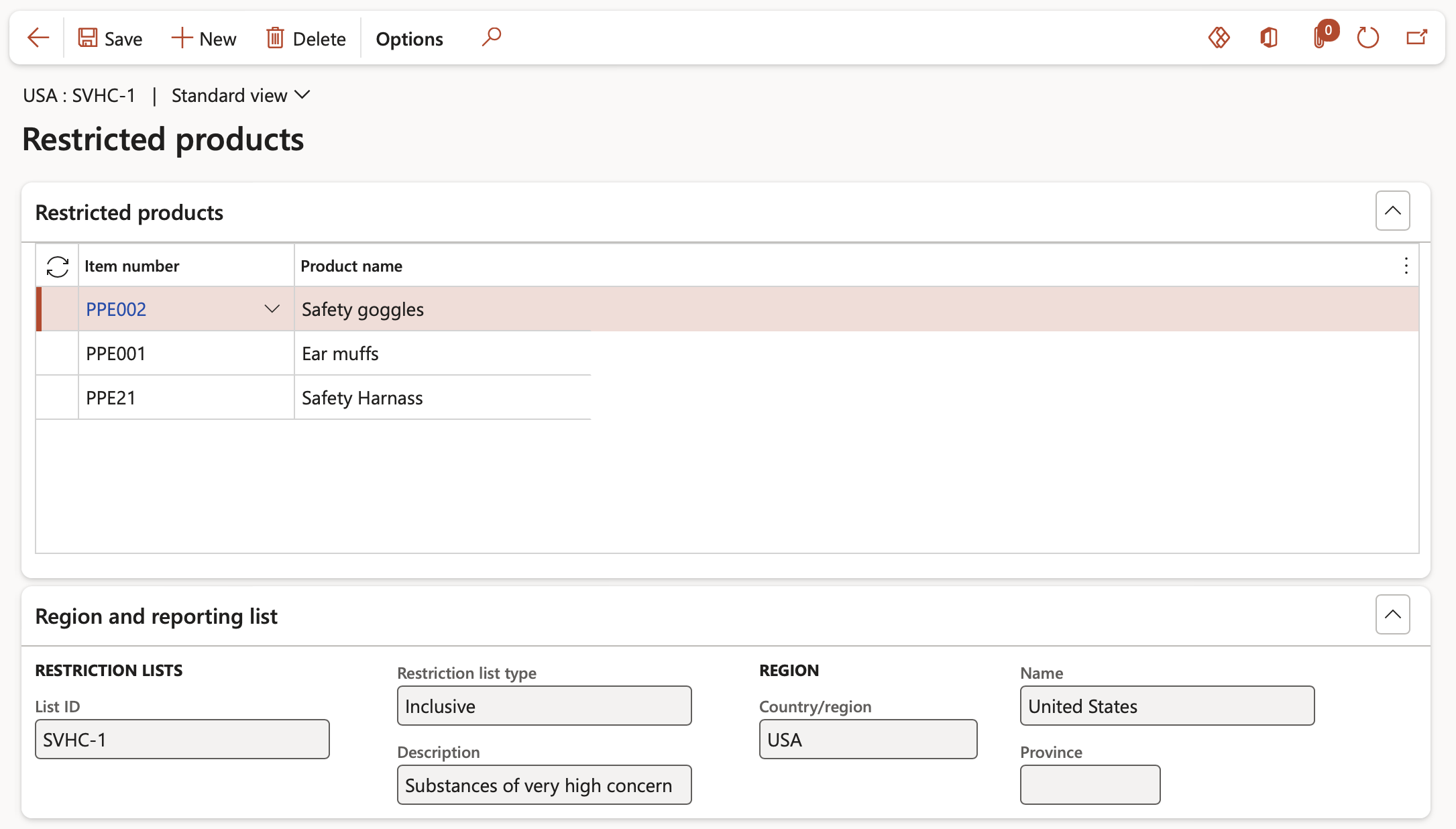
A product may be added to multiple exclusion lists for the same country/region but for differing states. However, a product may not also exist in an inclusive list for the same country/region.
Once added to lists, the items or products are handled by Dynamics 365 as restricted products.
If the item or product and an excluded region appear together on a sales order, Dynamics 365 will display an info log error and prevent the sale.

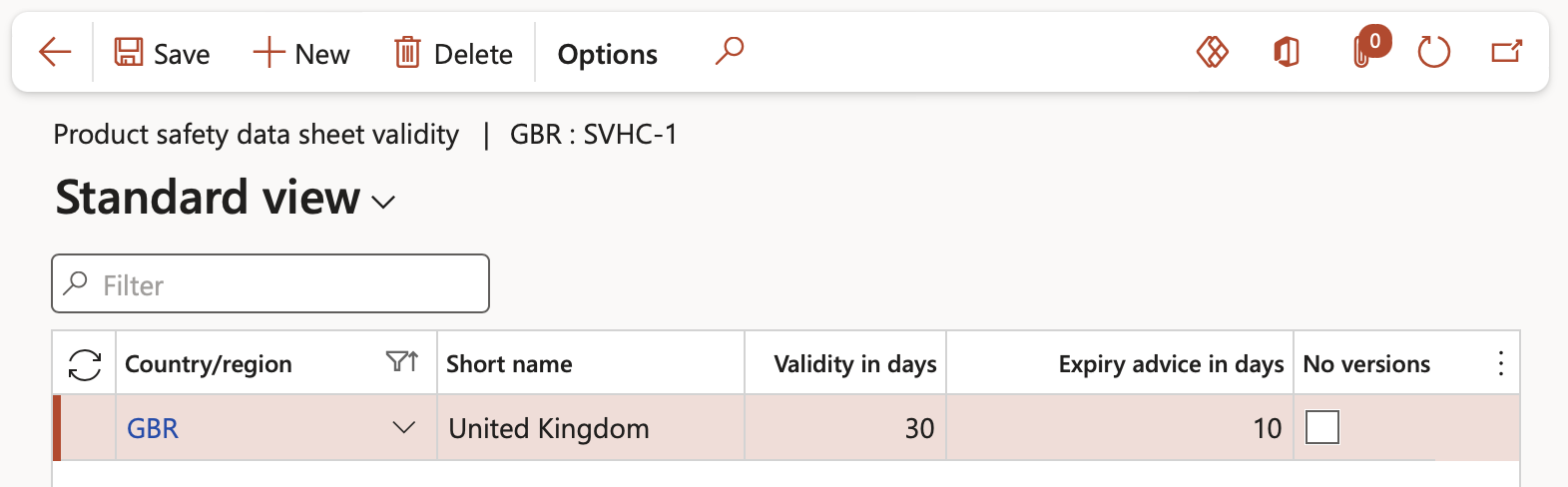
¶ Step 1.4: Set up Product safety data sheet for the Restricted product regional list
- Product safety sheet default data can be set up by selecting the applicable list and clicking the Product safety data sheet validity button on the Action pane

- On the Action pane, click on the New button
- In the Country/region field, select the relevant country from the dropdown list
- In the Validity in days field, enter the amount of days that the data sheet is valid for
- In the Expiry advice in days field, enter the number of days that the user should be advised before the data sheet expires
- Check the No versions check box to create a new document instead of versions for the purposes of resending the product safety data sheet. Product safety date sheet document versions are setup up per product from the Released products form covered in further down in this test script.
¶ Step 2: Setup Regulated and Reported products
Regulated products and reporting lists for countries and regions can be setup in Inventory management module. If the product is only regulated in a country or region then it need not be marked as reported. However, if usage data of a product needs to be reported then it needs to exist on a reporting list.
Go to: Product information management > Setup > Product compliance > Regulated products regional lists
- On the Action pane, click the New button
- In the Country/region field, select the relevant country from the dropdown list
- In the State/province field, select the relevant State from the dropdown list
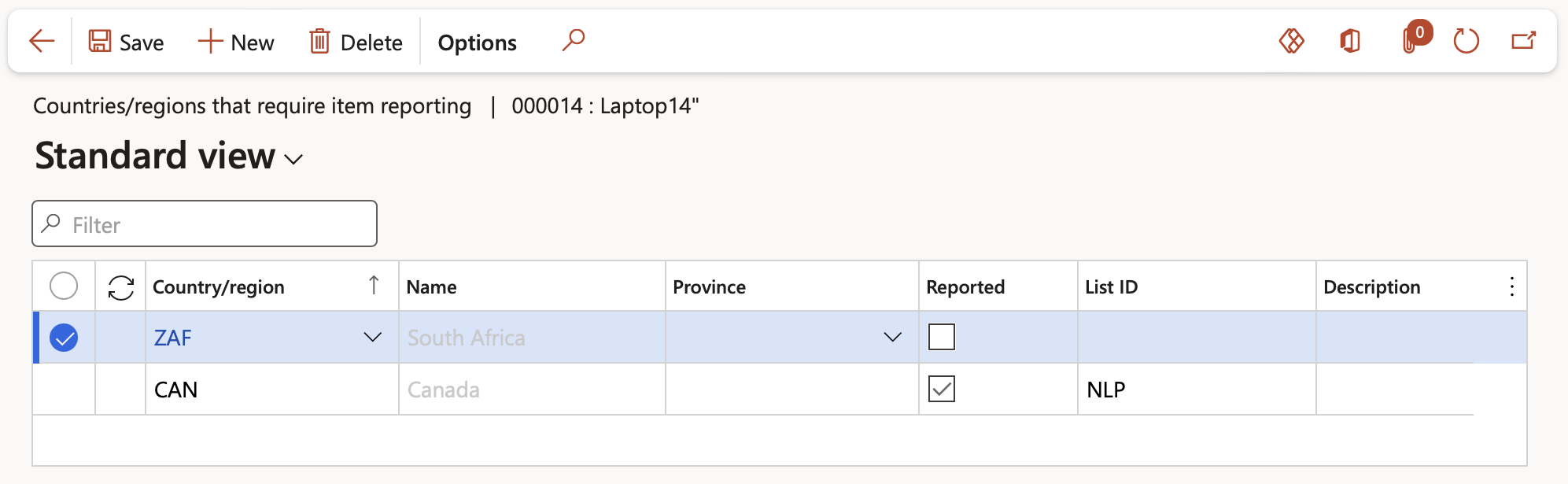
- If the Reported box is ticked, the product is regulated and usage information must be reported in compliance with regional and national authorities
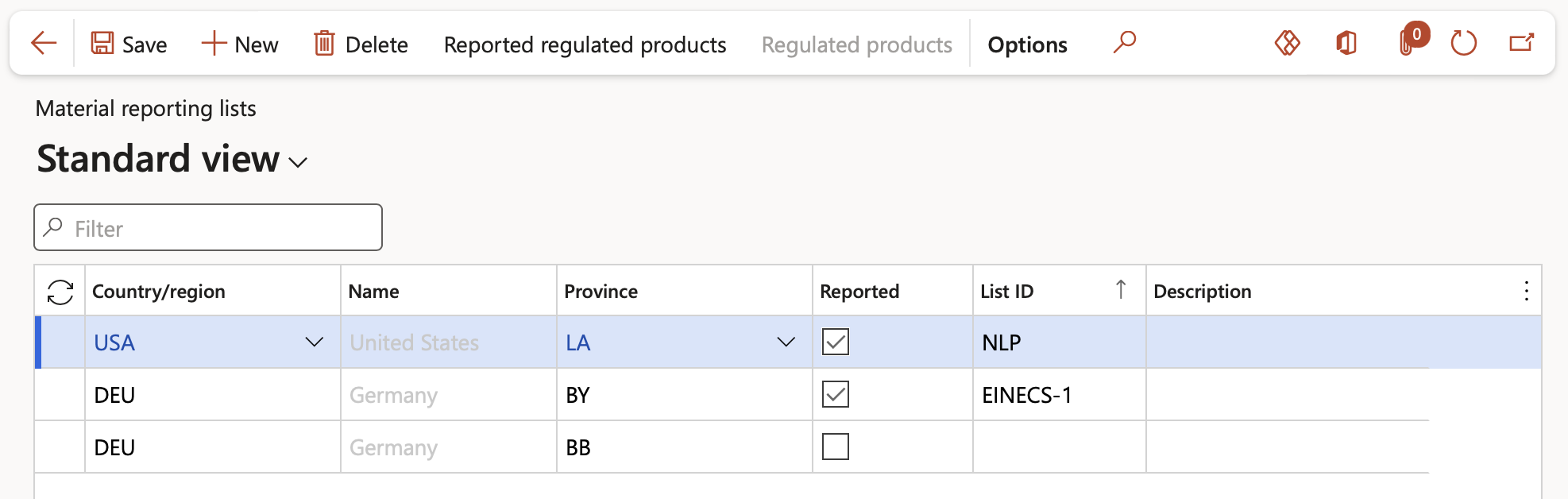
In the above screenshot, all products that are regulated in Germany but not reported can be setup against the first record in the table. All other records are for specific reported lists and hence will contain only products that need reporting in specific regions on given reporting lists
¶ Step 2.1: Adding of products to a Regulated product list
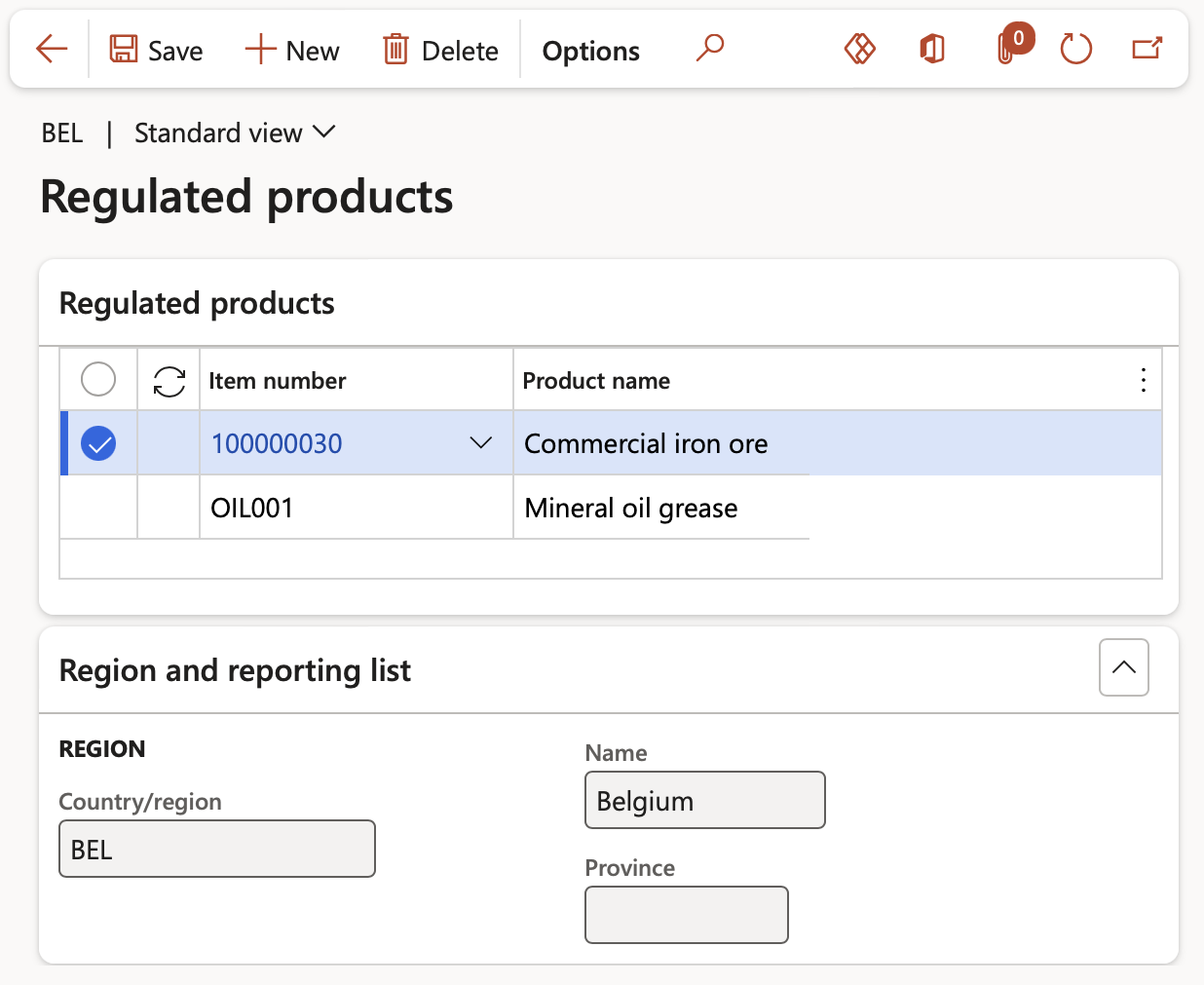
When the Reported tick box on a Regulated products list record has not been ticked, this record is not reported on and the Regulated products button is active from where products can be added to the list.
- On the Material reporting lists form, select a record with the Reporting tick box unticked and click the Regulated products button
- Click the New button and select the Item to be added to the Regulated products list in the Regulated products Fast tab. The details of the Regulated products list record to which the item is added is displayed in the Region and reporting list fast tab
¶ Step 2.2: Adding of products to a Reported regulated product list
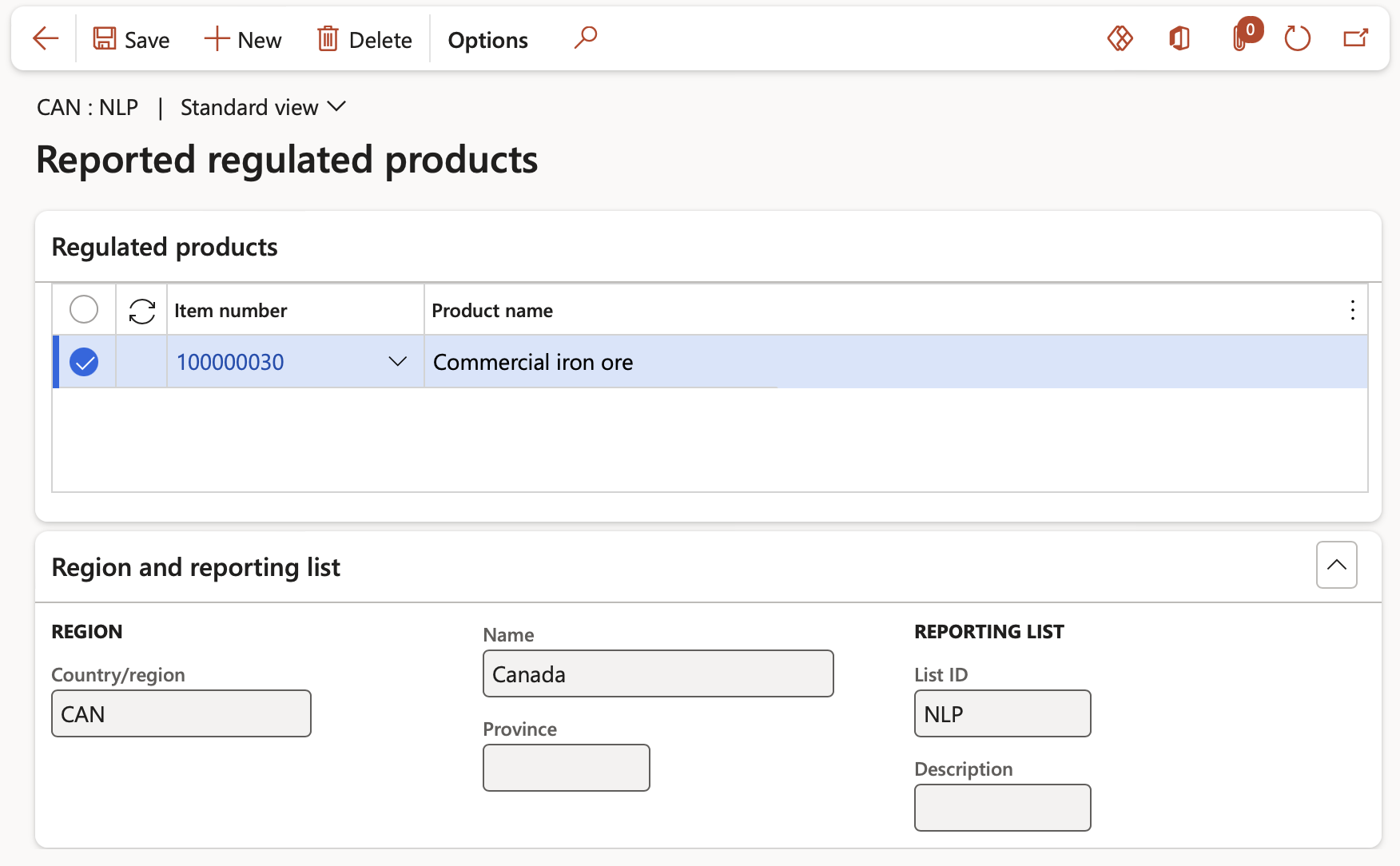
When the Reported tick box on a Regulated products list record has been ticked, this record is reported on and the Reported regulated products button is active from where products can be added to the list.
- In the Material reporting lists form, select a record with the Reporting tick box ticked and click the Reported regulated products button
- Click the New button and select the item to be added to the Reported regulated products list in the Regulated products Fast tab. The details of the Reported and regulated products list record to which the item is added is displayed in the Region and reporting list Fast tab
¶ Step 3: Product safety data sheet
Using this form, default parameters like validity interval and expiry advice interval for product safety data sheets can be setup
Go to: Product information management > Setup > Product compliance > Product safety data sheet validity
- On the Action pane, click on the New button
- In the Country/region field, select the relevant country from the dropdown list
- In the Validity in days field, enter the amount of days that the data sheet is valid for
- In the Expiry advice in days field, enter the number of days that the user should be advised before the data sheet expires
- Check the No versions check box to create a new document instead of versions for the purposes of resending the product safety data sheet. Product safety date sheet document versions are setup up per product from the Released products form covered in further down in this test script

¶ Step 4: Setup on Released products list page
Once standard lists are defined in the inventory management module, individual products can be added to such lists from the released products list page.
Go to: Product information management > Products > Released products
- Select the relevant Item on the list page
- On the Action pane, in the Compliance group, the following buttons are available:
Regulated products:
A Regulated or Regulated and reported list needs to exist for the specific item before it can be selected from this option. The user is only able to see the country/region in the list if a list is already defined for that item
¶ Step 4.1: Add an item to a Regulated product lists from the Released products form
Go to: Product information management > Products > Released products
- Select the relevant Item on the list page
- On the Action pane, in the Compliance group, click Regulated products
- Click the New button and populate all applicable fields from the values available in the dropdown fields for this product. The purpose of each field has been covered in Step 2 on the Regulated products regional lists form
Restricted products:
A Restricted list needs to exist before it can be selected from this option. The user is only able to select the list ID for a given country/region if a list for that combination is already defined. Using this option is faster if a product exists on multiple lists
¶ Step 4.2: Add an item to a Restricted product lists from the Released products form
Go to: Product information management > Products > Released products
- Select the relevant Item on the list page
- On the Action pane, in the Compliance group, click Restricted products
- Click the New button and populate all applicable fields from the values available in the dropdown fields for this product. The purpose of each field has been covered in Step 1.1 on the Restricted product regional list form
Safety data sheet:
Using this option, user can setup product safety data sheets in various languages and versions. The sheets can be activated as needed. If the records are changed, then a modification reason can be entered and an update log is automatically maintained
¶ Step 4.3: Set up the Product safety sheet for an item
Go to: Product information management > Products > Released products
- Select the relevant Item on the list page
- On the Action pane, in the Compliance group, click Safety data sheet
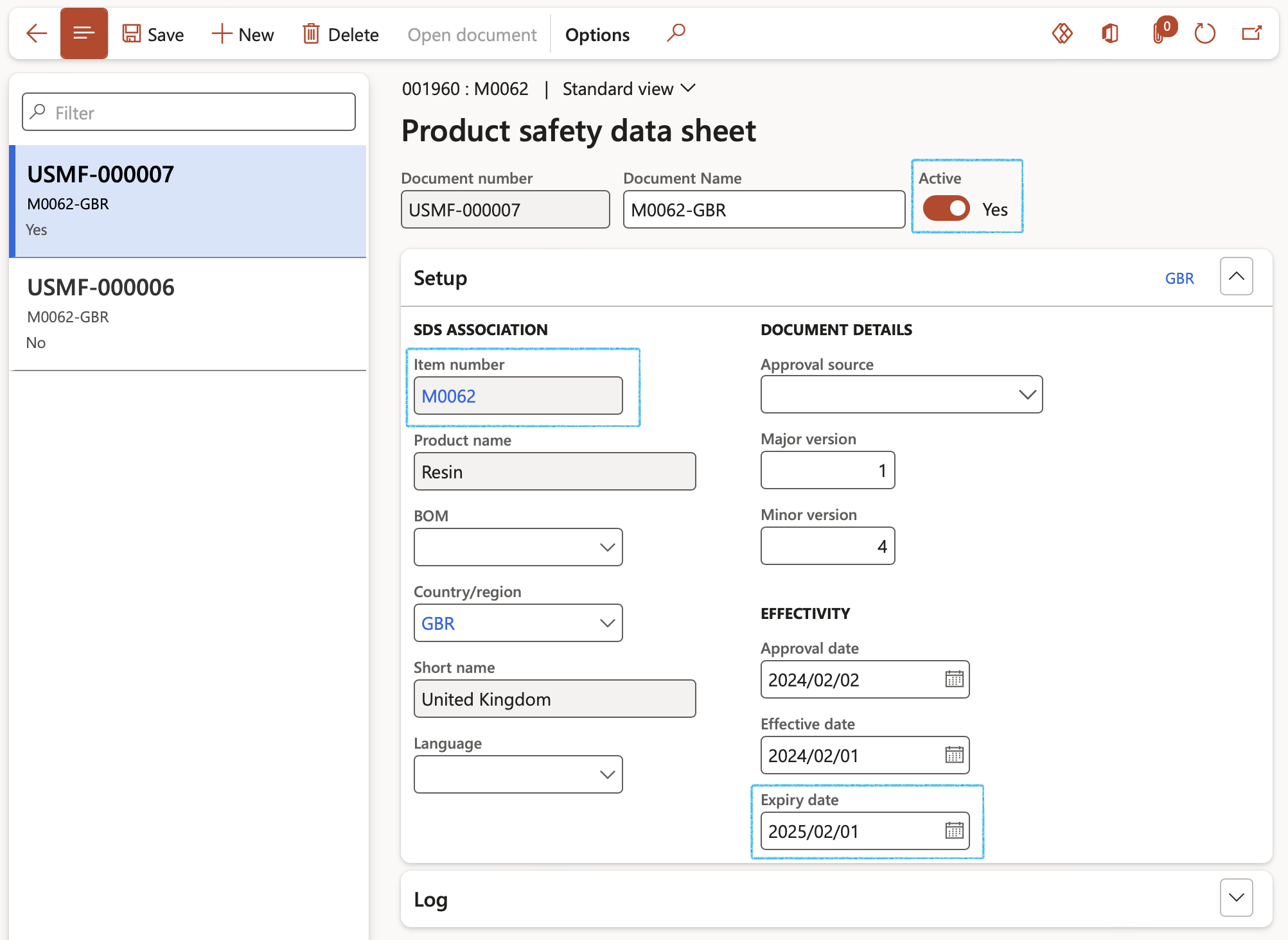
- The Product and safety data sheet form opens up with the unique Safety data sheet IDs for each document or versions in the left-hand margin, the Safety data setup in the Setup Fast tab and the logging details in the Log fast tab
- Click the New button to create a new product safety data sheet
- In the Document name field, enter a descriptive name for the product safety sheet
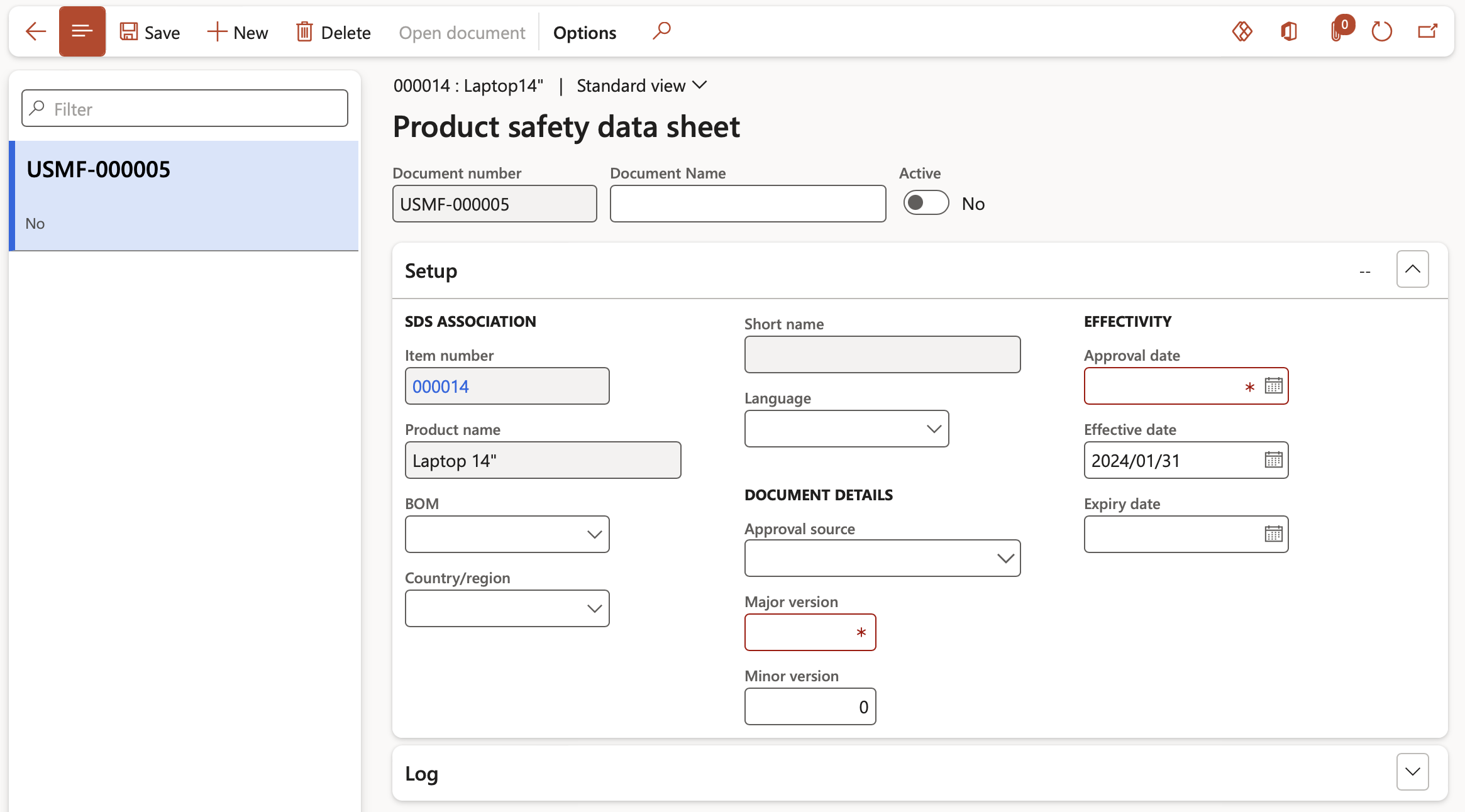
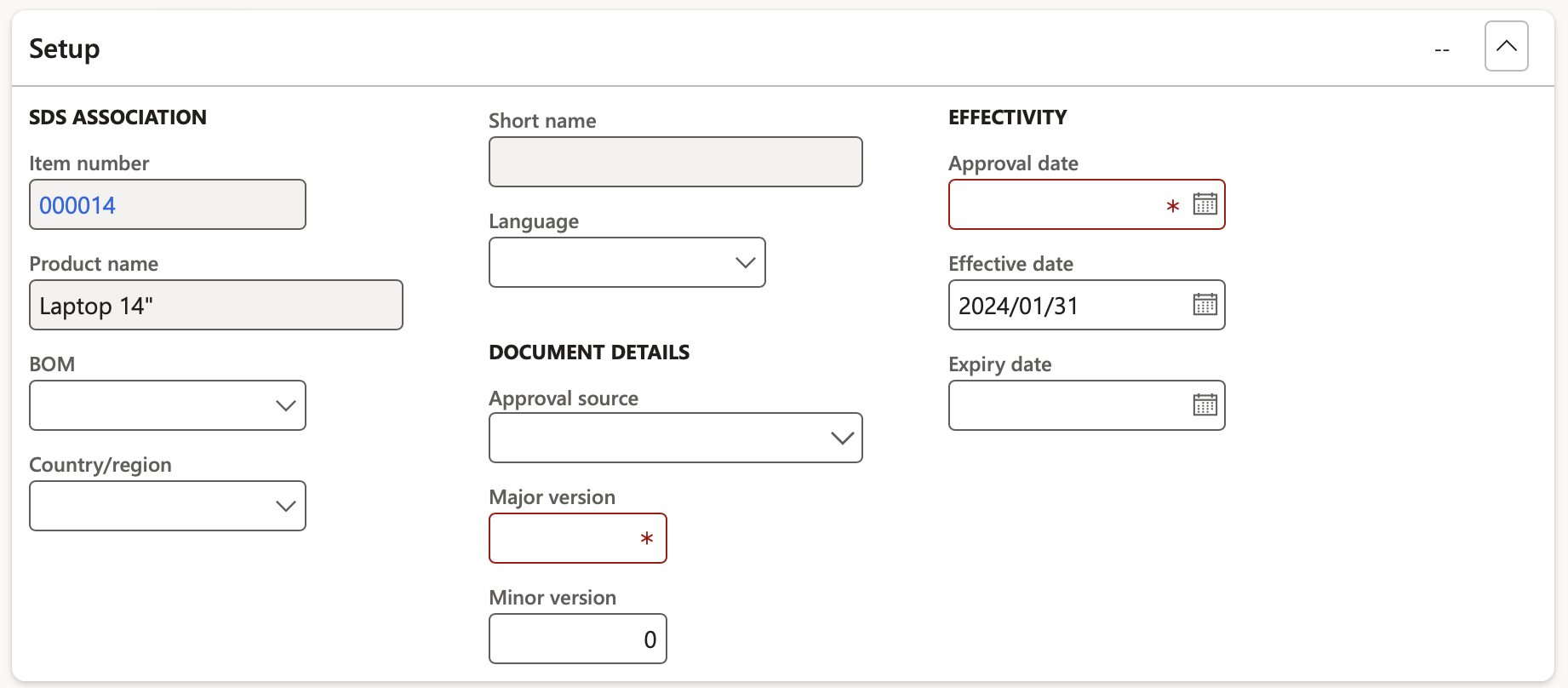
- Under the Setup fast tab the following fields can be populated:
- In the BOM field, select the Formula or Bill of materials that is associated to the product safety data sheet. If the field is left blank, the document applies to all formulas and BOMs.
- In the Country/region field, select country or region for which the product safety data sheet applies. If the field is left blank, the document applies to all countries and regions.
- In the Language field, select the language that is associated to the product safety data sheet. If the field is left blank, the document applies to all languages.
- In the Approval source field, select the employee who is responsible for approving the product safety data sheet.
- In the Major version and Minor version fields, specify the version numbers of this product safety data sheet.
- In the Approval date field, select the date on which the product safety data sheet has been approved. The approval date is used to calculate the validity interval of the product safety data sheet. The validity interval is the period that the document is valid.
- In the Effective date field, select the date from which the product safety data sheet is effective.
- In the Expiry date field, select the date on which the product safety data sheet expired. If the field is left blank, the document does not expire.
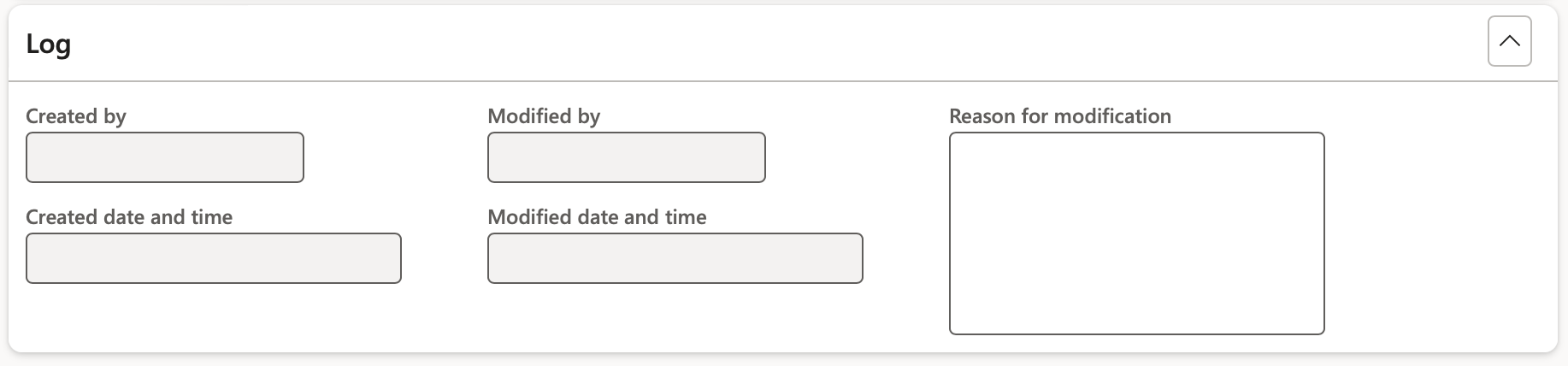
- Under the Log Fast tab the following fields can be populated:
- In the Reason for modification field, the reason for modifying or updating the product safety data sheet can be provided.
- The Created by, Modified by, Created date and time and Modified date and time fields are auto populated to indicate who created or updated the product safety data sheet and when
There are several parameters in inventory module that control the display and timing of alerts specific to events that may occur related to product safety data sheets
Reporting details:
Using this form the user can setup additional information as required by some US-centric regulatory authorities. This information can be printed to documents or exposed to external interfaces as maybe needed. If a substance name as defined by a regulatory body is referenced from an external system, then all the product related information attached to such a name can be retrieved
¶ Step 4.3.1: Set up the Reporting details for an item
Go to: Product information management > Products > Released products
- Select the relevant Item on the list page
- On the Action pane, in the Compliance group, click Reporting details
- The Reporting details form opens with a Compliance data and a Usage data fast tab. Click on the Edit button to update the fields
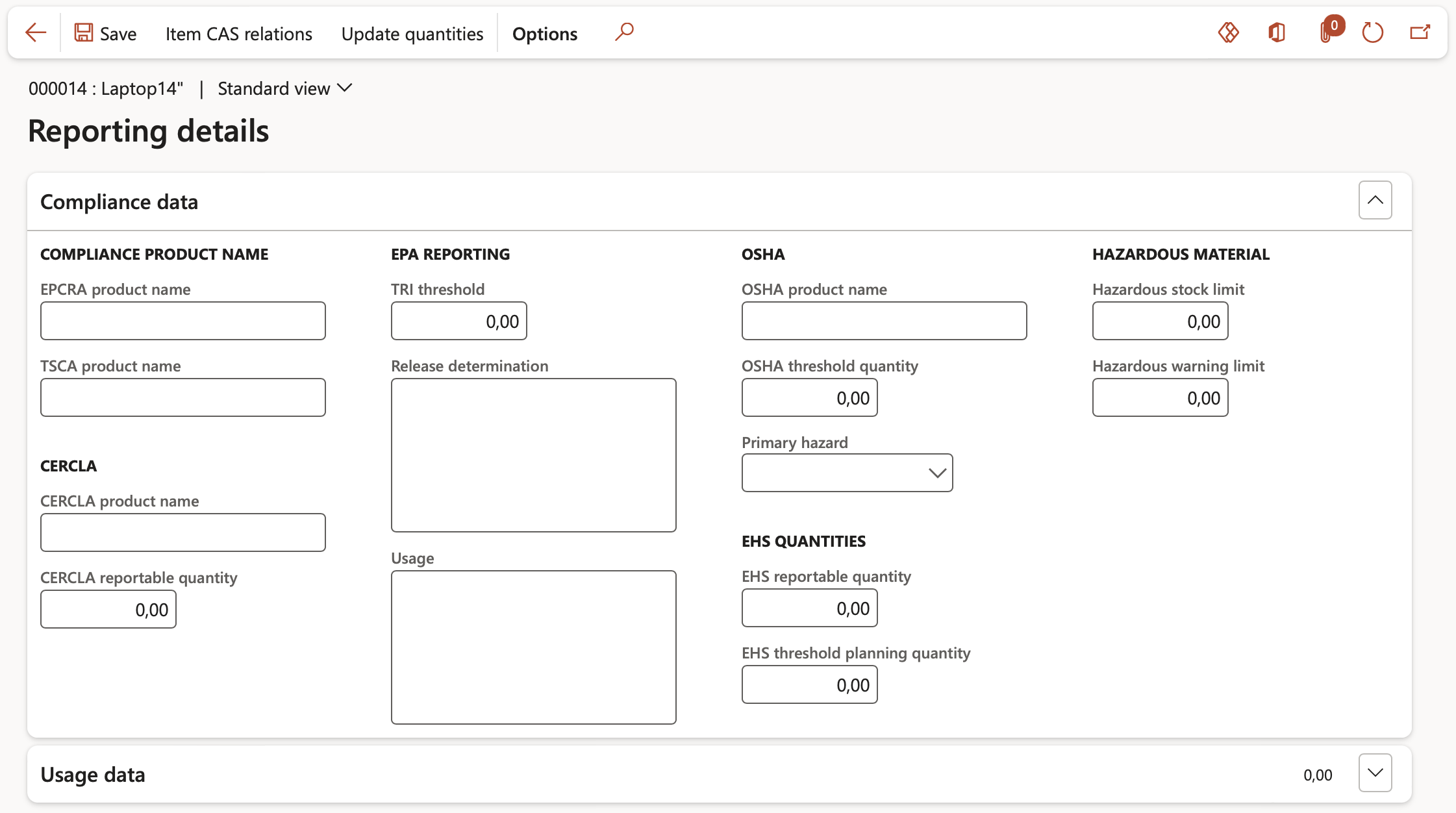
- Under the Compliance data fast tab, the following Regulatory authorities are covered by this form:
- EPCRA: Emergency, Planning and Community Right-To-Know Act
- TSCA: Toxic Substances Control Act
- CERCLA: Comprehensive Environmental Response, Compensation, and Liability Act
- In the CERCLA reportable quantity field, specify the quantity to be reported on as governed by the CERCLA regulatory authority.
- TRI: Toxics release Inventory from United States Environmental Protection Agency
- In the TRI threshold field, specify the threshold quantity to be reported on as governed by the TRI regulatory authority.
- In the Release determination field, specify the text that describes the release of TRI.
- In the Usage field, specify the text that describes the use of TRI.
- OSHA: Occupational Health and Safety Administration
- In the OSHA threshold quantity field, specify the threshold quantity to be reported on as governed by the OSHA regulatory authority.
- In the Primary hazard field, select the relevant hazard from the dropdown.
- EHS: Extremely Hazardous Substances
- In the EHS reportable quantity field, specify the quantity at which reporting is required as governed by the EPCRA regulatory authority.
- In the EHS threshold planning quantity field, specify the EHS threshold quantity at which an emergency plan must be prepared as governed by the EPCRA regulatory authority
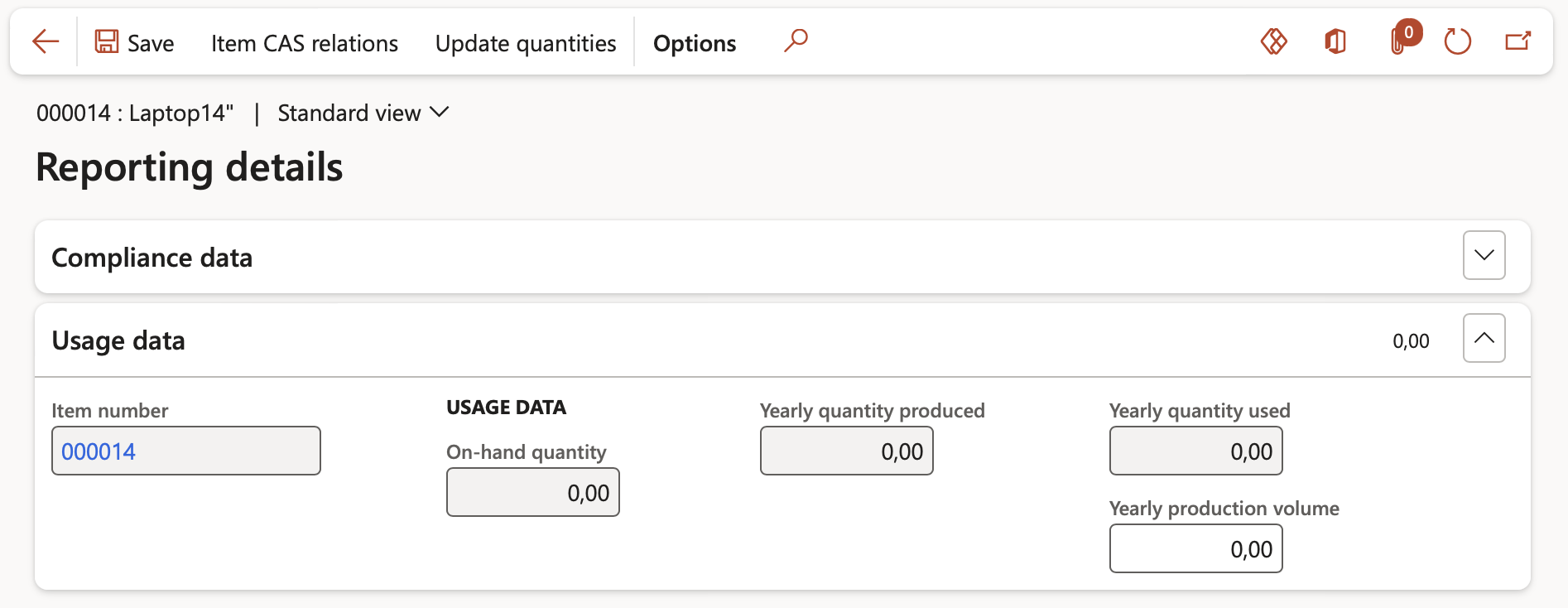
- Under the Usage data Fast tab, the user can check the usage data for a particular product. Such information can then either be exposed to custom reports or provided to external systems electronically. CAS numbers (Chemical Abstract Registry) can be linked to the product on this form.
- Under the Usage data fast tab, the following data can be seen and populated:
- In the Item number field, the item number related to the Reporting details set up can be seen.
- In the On-hand quantity field, the on hand quantity of the item number can be seen.
- In the Yearly quantity produced field, the quantity of this item that was produced each year (manufacturing) can be seen. The period for which this field is calculated is as per the Start and End date fields in the Annual reporting quantity basis field group in the Inventory and warehouse management parameters >Product compliance tab.
- In the Yearly quantity used field, the quantity of this item that was used/consumed each year can be seen. The period for which this field is calculated is as per the Start and End date fields in the Annual reporting quantity basis field group in the Inventory and warehouse management parameters >Product compliance tab.
- In the Yearly production volume field, the planned production volume for the year can be specified
¶ Step 4.3.2: Set up Item CAS relations to Reporting detail records:
Go to: Product information management > Products > Released products
- Select the relevant Item on the list page
- On the Action pane, in the Compliance group, click Reporting details
- On the Reporting details form, in the Action pane, click on the Item CAS relations button
- The Item CAS relations form opens. To add a record to the form, click the New button and populate the following fields:
- In the EC number field, enter the European Community number with format ###-###-#
- In the CAS number field, capture the Chemical Abstract Service identification number
- In the CAS name field, capture the Chemical Abstract Service name
- In the Weight % field, specify the applicable weight percentage
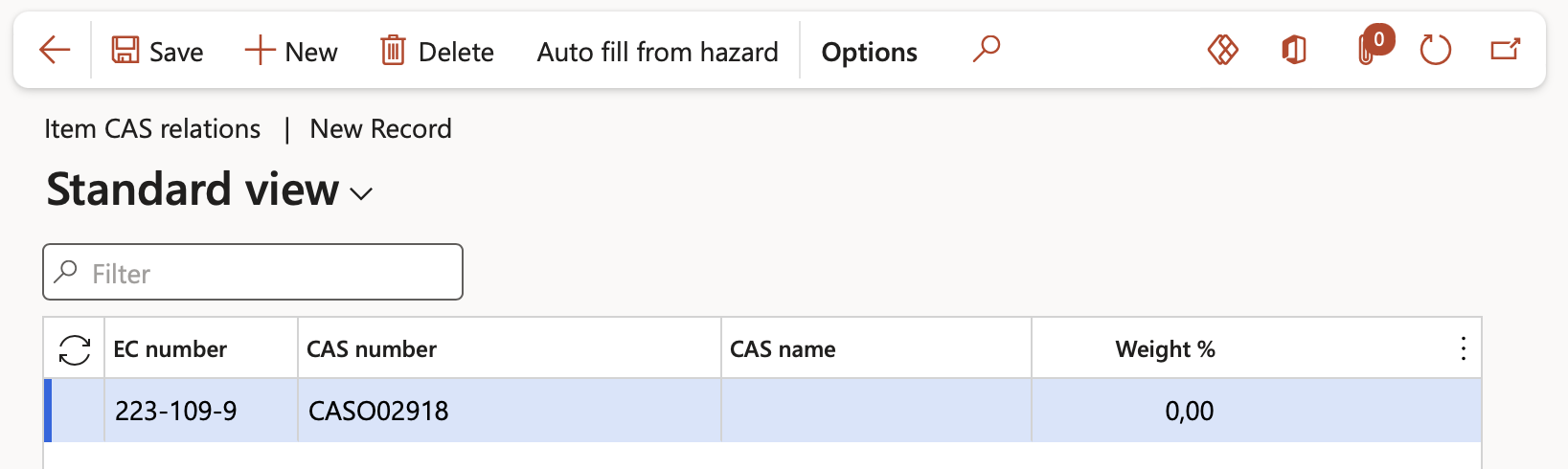
The Auto fill from hazard button can be used to auto-populate the Item CAS relations form if the item is already linked to an Hazard
¶ Step 5: Setting up product compliance parameters
As shown in the screenshot below, parameters in the Inventory management module can be setup to receive warnings and/or e-mail notifications during following processes:
- Purchase order entry
- Sales order entry
- Sales packing slip posting
- Sales invoice posting
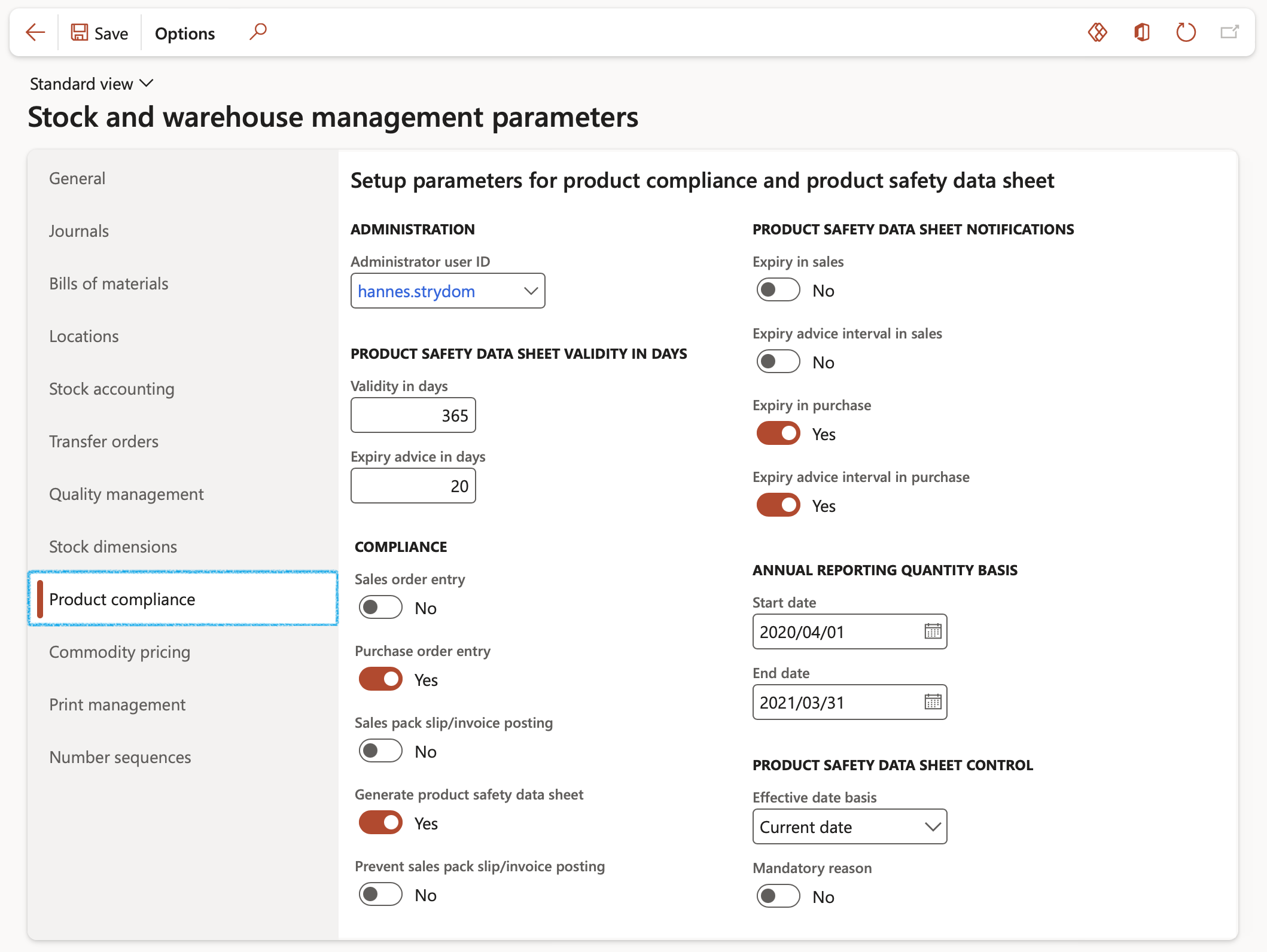
Go to: Stock management > Setup > Stock and warehouse management parameters
- The following fields can be set and populated on the Product compliance tab:
- In the Administrator user ID field, select the user identification for the product compliance administrator. This user receives the notifications that you select in the Product safety data sheet notifications group.
- In the Product safety data sheet validity in days group, set up the following parameters:
- Validity in days – Enter the default number of days that product safety data sheets are valid from their approval date.
- Expiry advice in days – Enter the number of days before the expiration date of product safety data sheets that users are notified of the pending expiration.
- In the Compliance group, select the options that are required to issue user warnings when product safety data sheets are expired or were never sent to the customer. You can decide to print these documents at the time when packing slips are printed to provide to customers who never received the documents. You can also prevent the posting of packing slips and invoices when the documents are expired.
- In the Product safety data sheet notifications group, select the options for issuing alerts and warnings to the product compliance administrator. This is done when product safety data sheets are either expired or were never sent to the customer. You can also select printing and posting parameters
- In the Annual reporting quantity group, select the start and end dates used to calculate annual quantities for reporting usage data
- In the Product safety data sheet control group, select the option to require users to specify a reason when product safety data sheets are modified. You also select the basis to use for determining the effective date for product safety data sheets when sales order and purchase order transactions are generated. You can select from the following options:
- Current date — Use the product safety data sheet that is in effect on the date that the sales order or purchase order is entered as the active document
- Delivery date — Use the product safety data sheet that is in effect on the requested delivery date for the sales order or purchase order as the active document. This is the date on which the sales order or purchase order is expected to ship
¶ Daily use
In this section the user will be taken through the scenarios by the way of stepping through Sales order and Purchase order examples to demonstrate how the setup performed in the Setups section above are utilized in day to day processes.
¶ Scenario 1: Restricted product sold in a restricted state
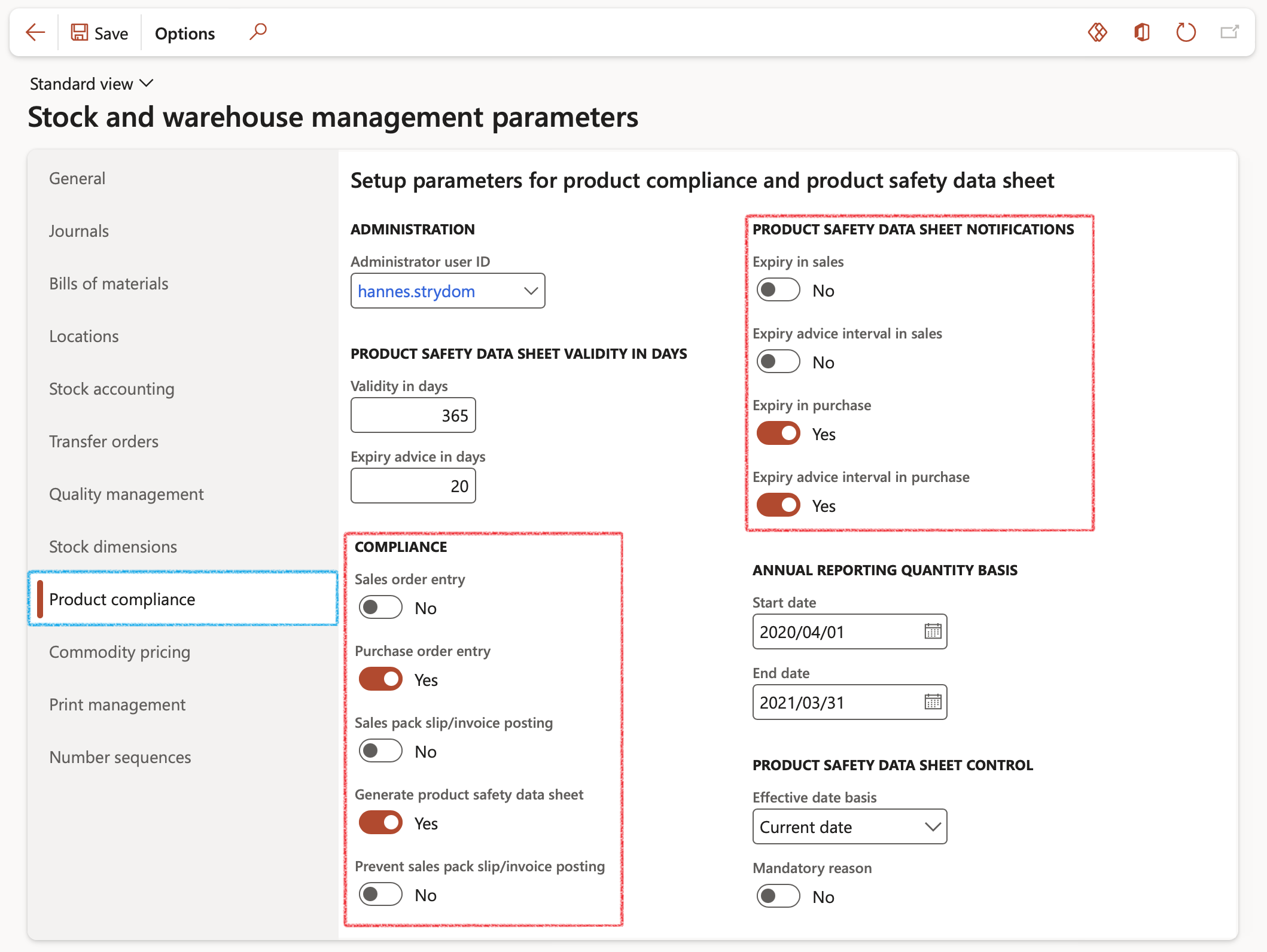
- On the setup of the Inventory and warehouse management parameters form below, it can be seen that all Compliance and Product safety data sheet notification sliders boxes been set to Yes in order to display how these checks are applied to the Sales order process
- In this scenario the EN state of GBR has been set as an Exclusive restriction type restricted product regional list
Go to: Product information management > Setup > Product compliance > Restricted products regional lists
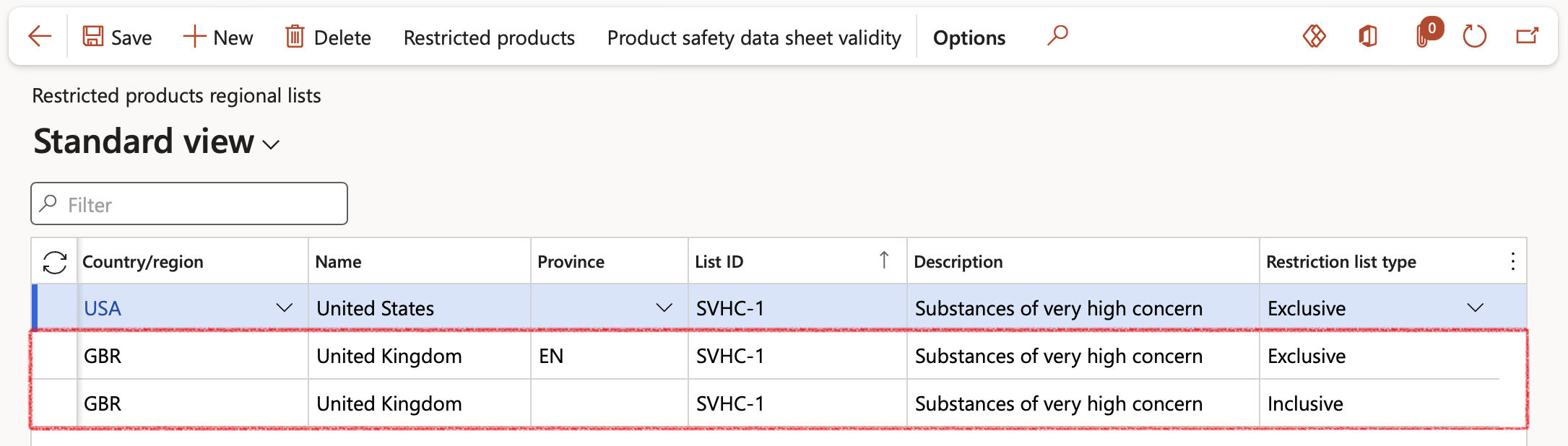
- The M0062 item has been selected and linked to the EN state in GBR restricted product regional list record. This means that all sales of item M0062 made to any customer with delivery address in the EN state will be blocked at all the Sales order stages activated in the Inventory and warehouse management parameters form above.

Go to: Sales and marketing > Sales orders > All sales orders
- Create a new Sales order and select a customer and delivery address in the EN state in GBR as shown below.
- Now click on the Add line button in the Sales order lines Fast tab and add item M0062 in the Item number field.
- Also populate the following fields on the Sales order line:
- Quantity
- Unit
- Unit price
- Site (Add through the Sales order line button > Dimension display option of the field does not appear on the Sales order line)
- Warehouse (Add through the Sales order line button > Dimension display option of the field does not appear on the Sales order line)
- Click the Save button
- The red error message appears as expected informing the user that item M0062 is restricted for sale to the selected delivery address.
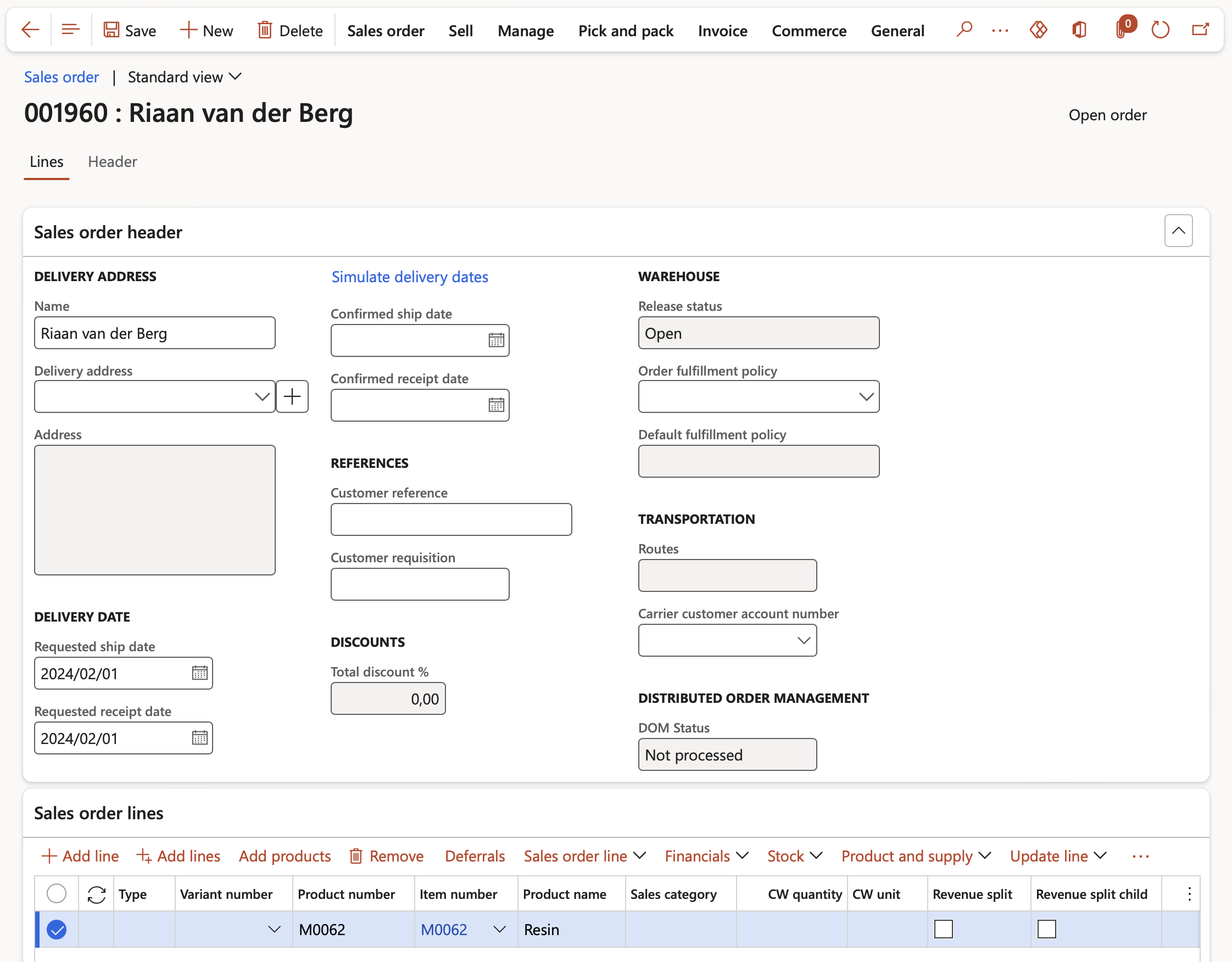
- From the Sales order line > Stock button, the Product safety data sheet log can be updated as well as the Safety data sheet accessed and printed if required.
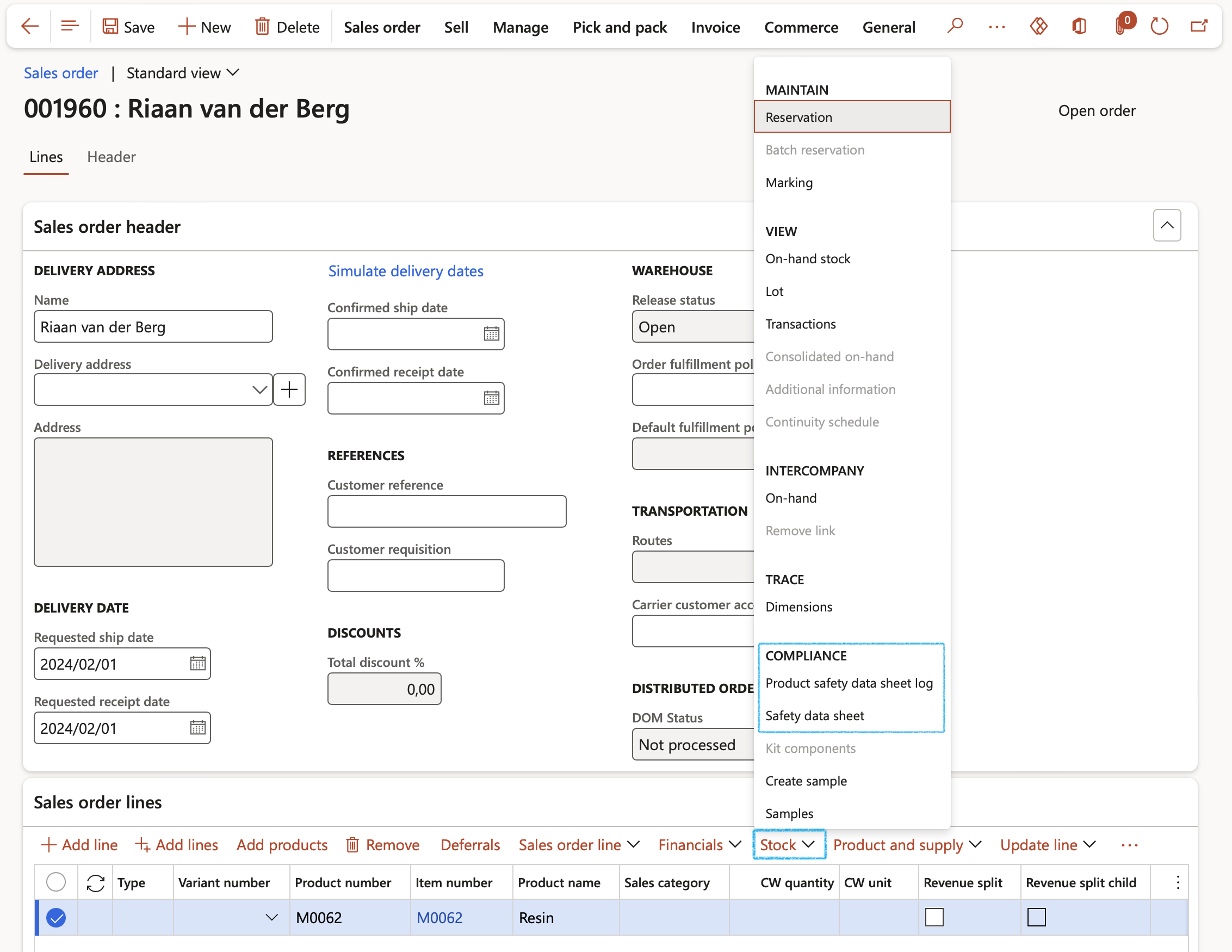
- When the user clicks the Sales order line > Stock button > Product safety data sheet log button, the Product safety data sheet log form opens. If a product is regulated or restricted and if a product safety data sheet already exists then it would be an existing record and accessible upon sales order (or purchase order) entry in the Product safety data sheet log form. If a new or existing data sheet is to be sent to a customer then that can be added to the log as well. This form keeps a log of all safety data sheets sent or received on each order.
- Add a new line by clicking the New button and populated the relevant field. All these fields are also found on the Product safety data sheet form.

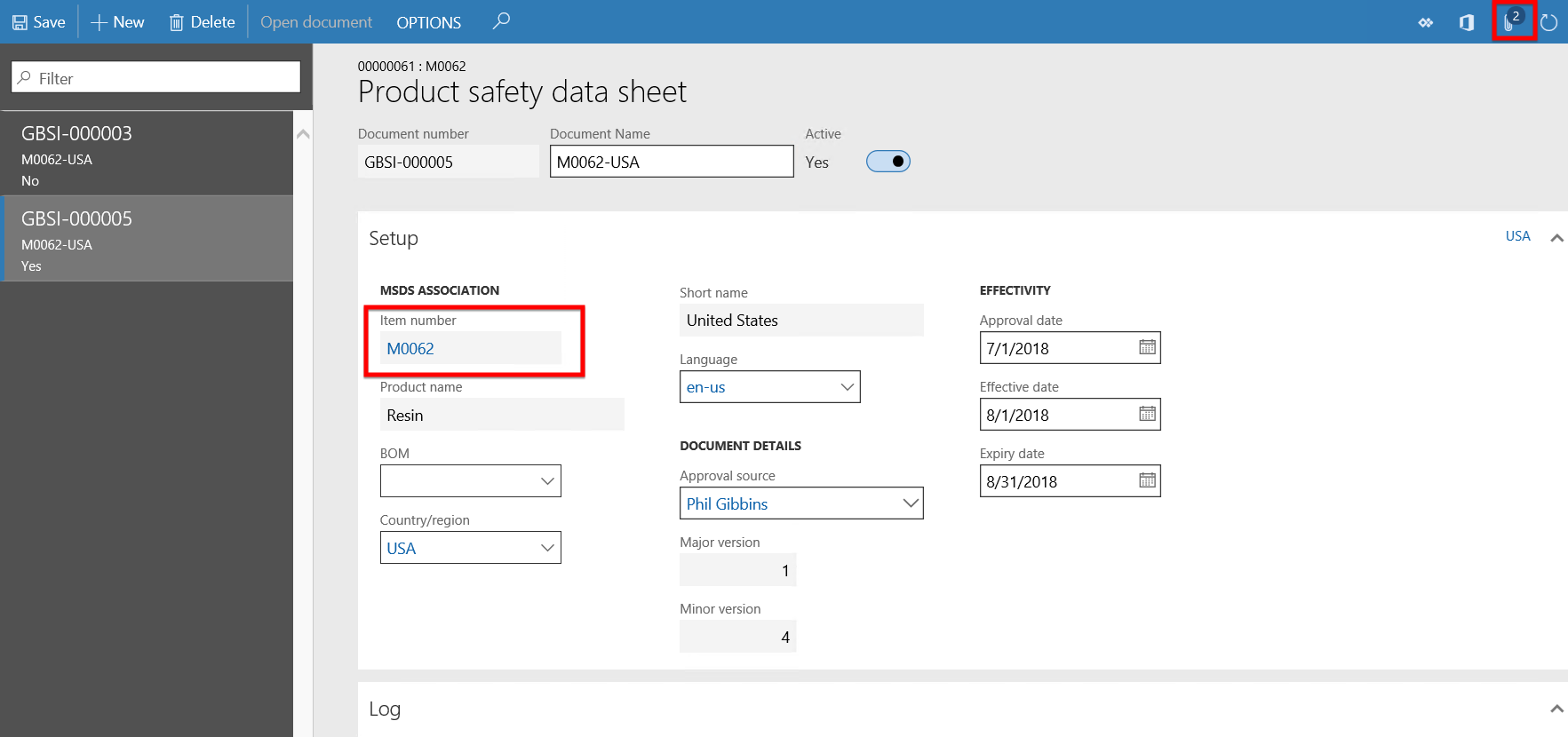
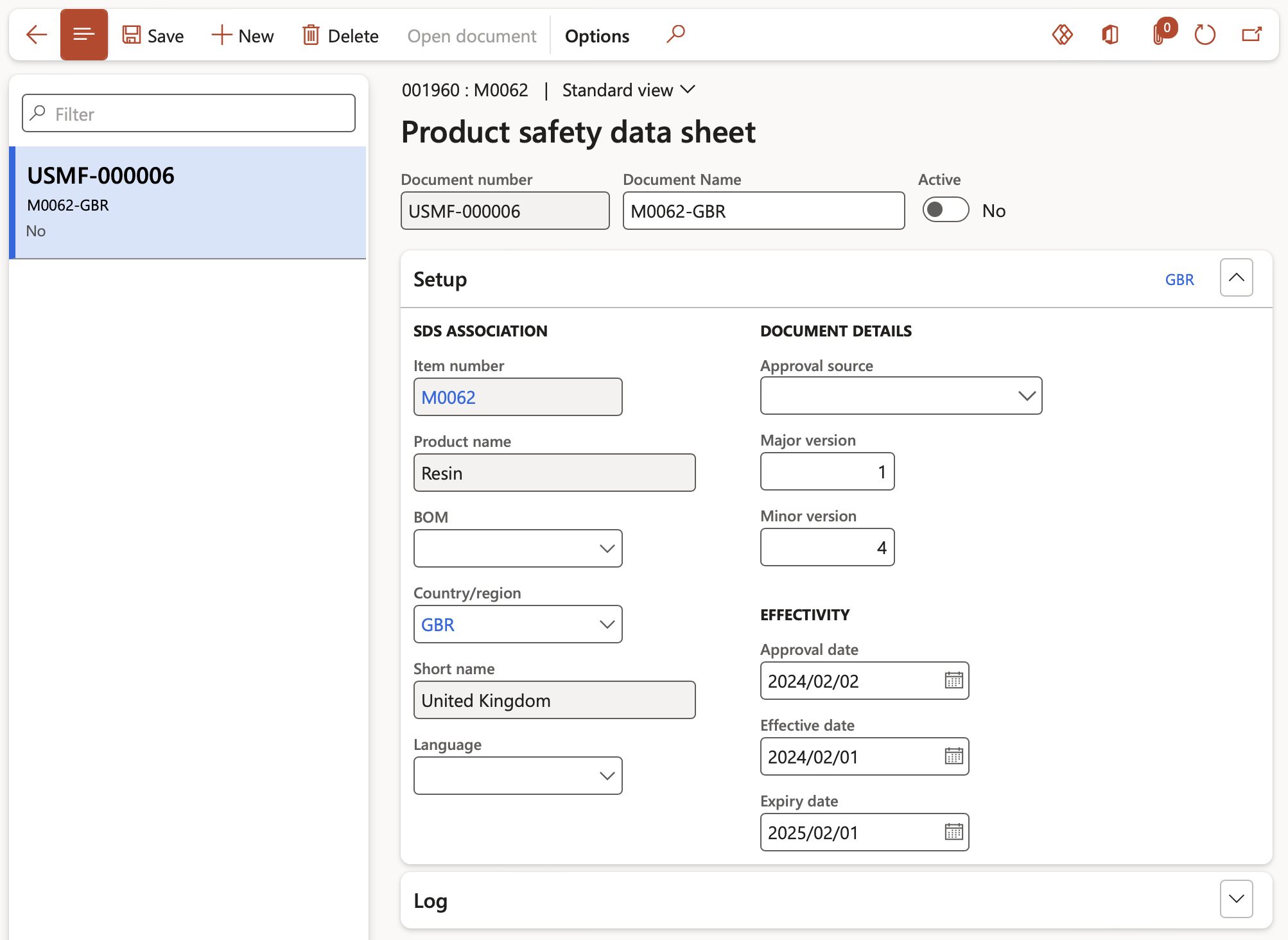
- When the user clicks the Sales order line > Inventory button > Safety data sheet button, the Product safety data sheet form opens.
- From this form the Product safety data sheet details can be reviewed, updated or opened from Document handling and printed if required.
¶ Scenario 2: Restricted product with expired Product safety sheet sold in a non-restricted state
Continuing with the same product M0062 and parameter setup as in Scenario 1, but in this scenario creating a Sales order for a different customer or the same customer with a delivery address in state where the product is not restricted. As can be seen in the active Product safety data sheet of product M0062 below, the Expiry date field is a date older than today’s date which means this safety sheet has expired.

Go to: Sales and marketing > Sales orders > All sales orders
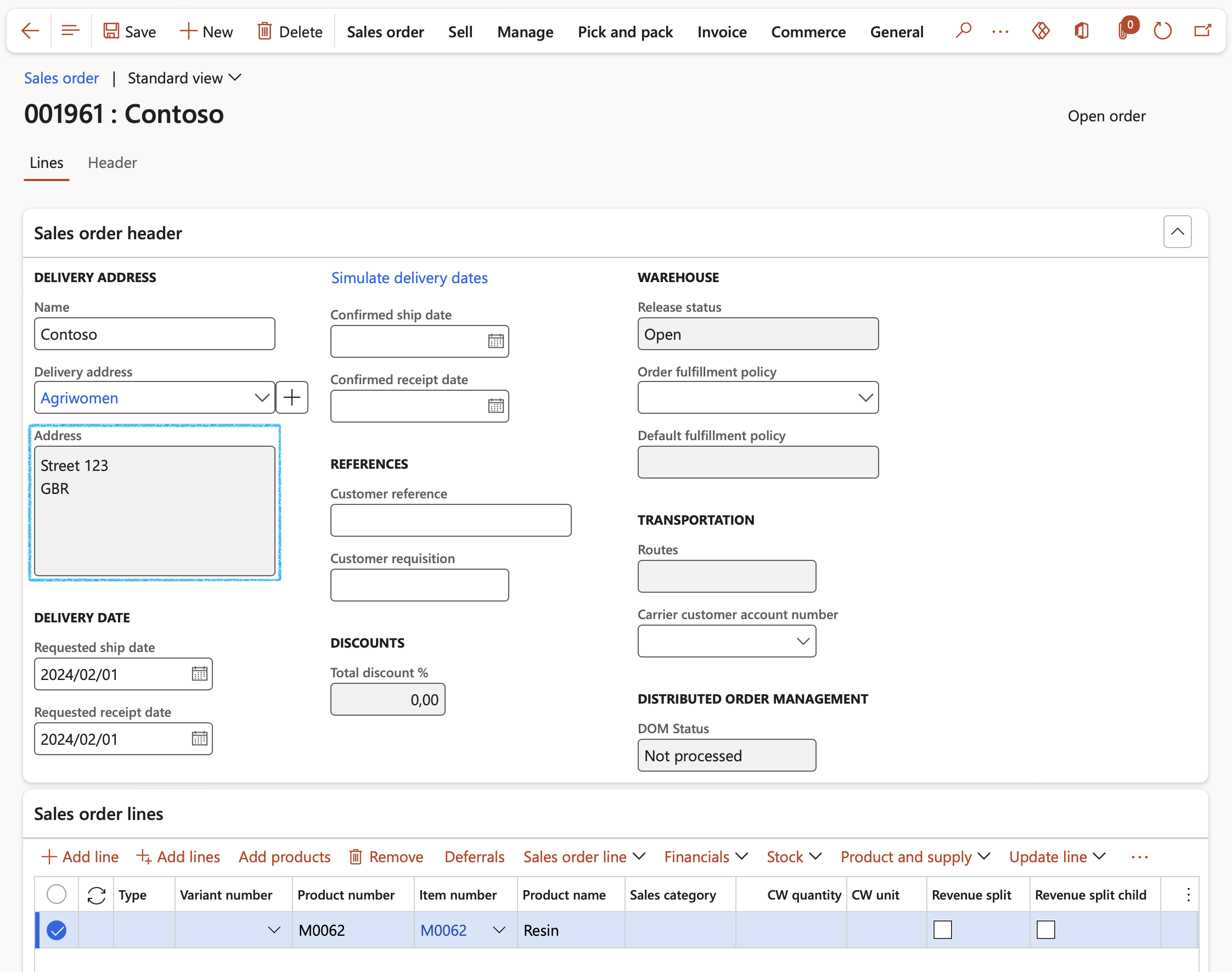
- Create a new Sales order and select a customer and delivery address that is in a non-restricted state, i.e. not the EN state in GBR as shown below.
- Now click on the Add line button in the Sales order lines Fast tab and add item M0062 in the Item number field.
- Also populate the following fields on the Sales order line:
- Quantity
- Unit
- Unit price
- Site (Add through the Sales order line button > Dimension display option of the field does not appear on the Sales order line)
- Warehouse (Add through the Sales order line button > Dimension display option of the field does not appear on the Sales order line)
- Click on the Save button
- As the delivery address is not to a restricted state, the red error message that appeared in Scenario 1 does not come up again.
- As can be seen below, a different warning message appears warning the user that the latest product safety sheet needs to be delivered to the customer for this product.
- On the Sales order form, go to Pick and pack > Post packing slip.
- The Packing slip posting form opens and the two fields under the COMPLIANCE DOCUMENTS field group on the Parameters Fast tab is copied from the Product compliance settings on the Inventory and warehouse management parameters form.
- Set the Quantity field to All and click the OK button to post the packing slip for item M0062 with the expired Product safety sheet. As the Prevent sales pack slip/invoice posting field is set to Yes, the expectation is that the Packing slip posting is prevented.
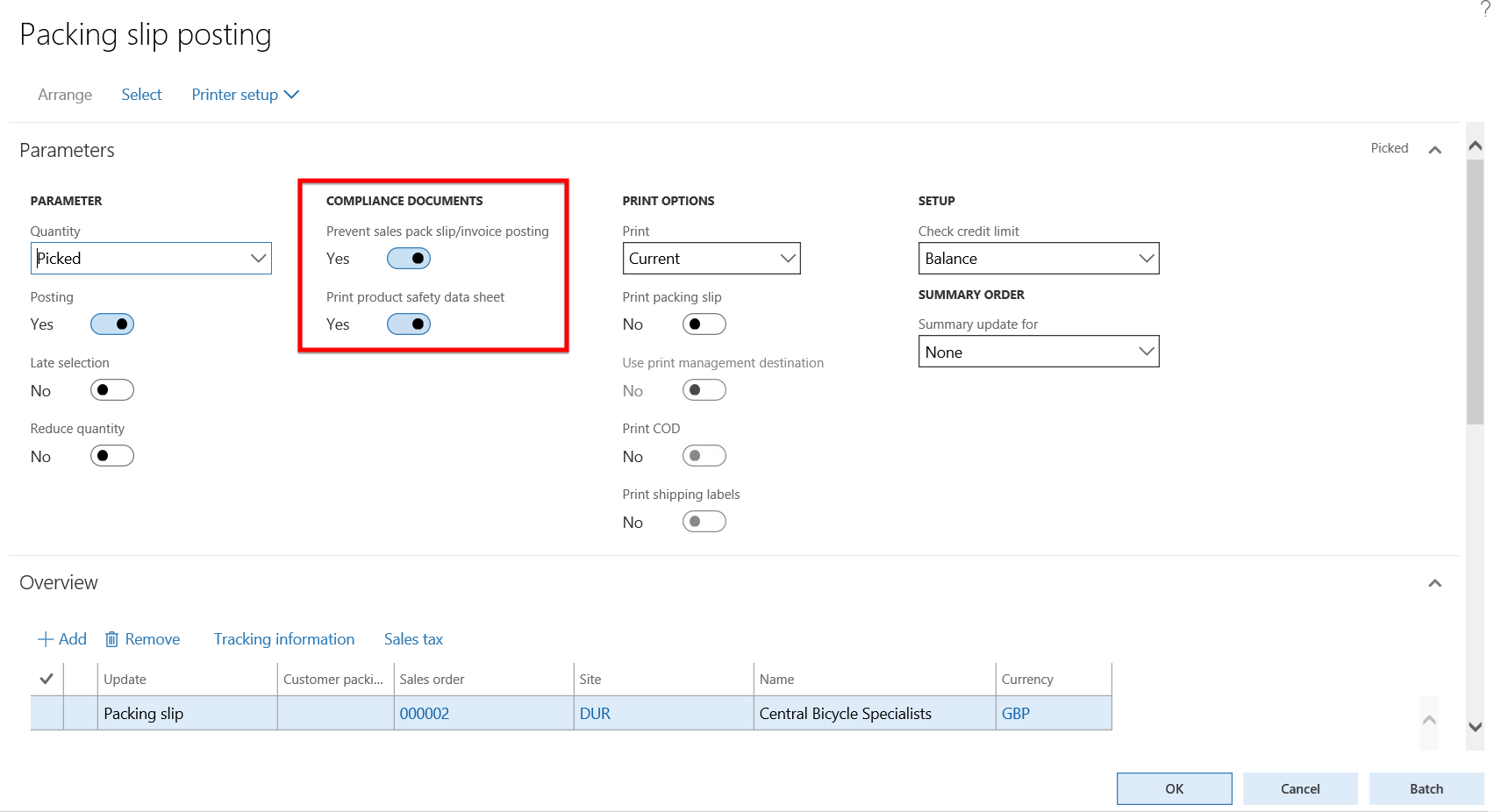
- An error message appears on the Sales order form stating that:
- The latest product safety sheet needs to be delivered to the customer for this product and
- No valid product safety sheet exists for the item on the sales order.
- The Packing slip posting has thus been prevented due to the expired product safety data sheet.
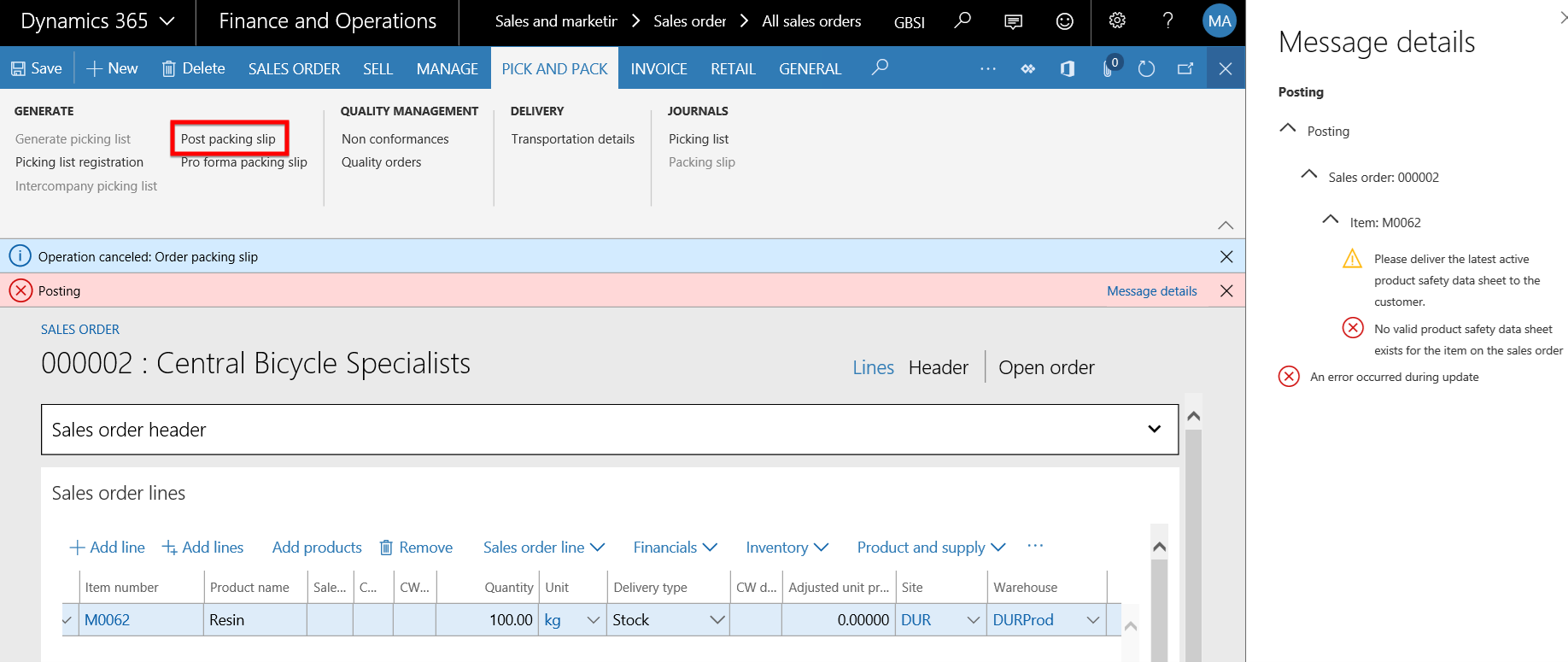
- Follow the same steps as in the Packing slip posting step above, but this time set the Prevent sales pack slip/invoice posting field is set to No and post the Packing slip.
- The Packing slip posts successfully and only the warning message to that the latest product safety sheet needs to be delivered to the customer for this product is displayed to the user.
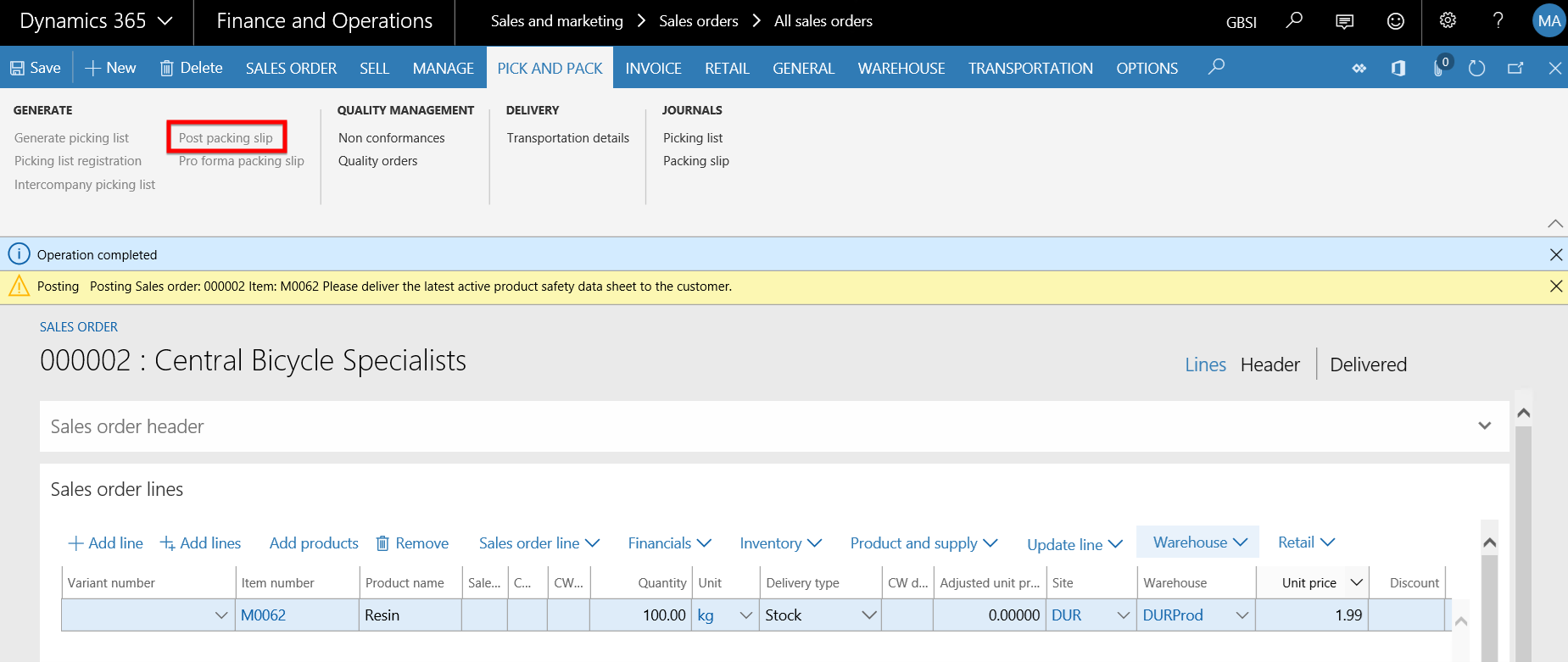
- On the Sales order form, go to INVOICE > Invoice.
- The Posting invoice form opens and the two fields under the COMPLIANCE DOCUMENTS field group on the Parameters Fast tab is copied from the Product compliance settings on the Inventory and warehouse management parameters form.
- Set the Quantity field to All and click the OK button to post the invoice for item M0062 with the expired Product safety sheet. As the Prevent sales pack slip/invoice posting field is set to Yes, the expectation is that the invoice posting is prevented.
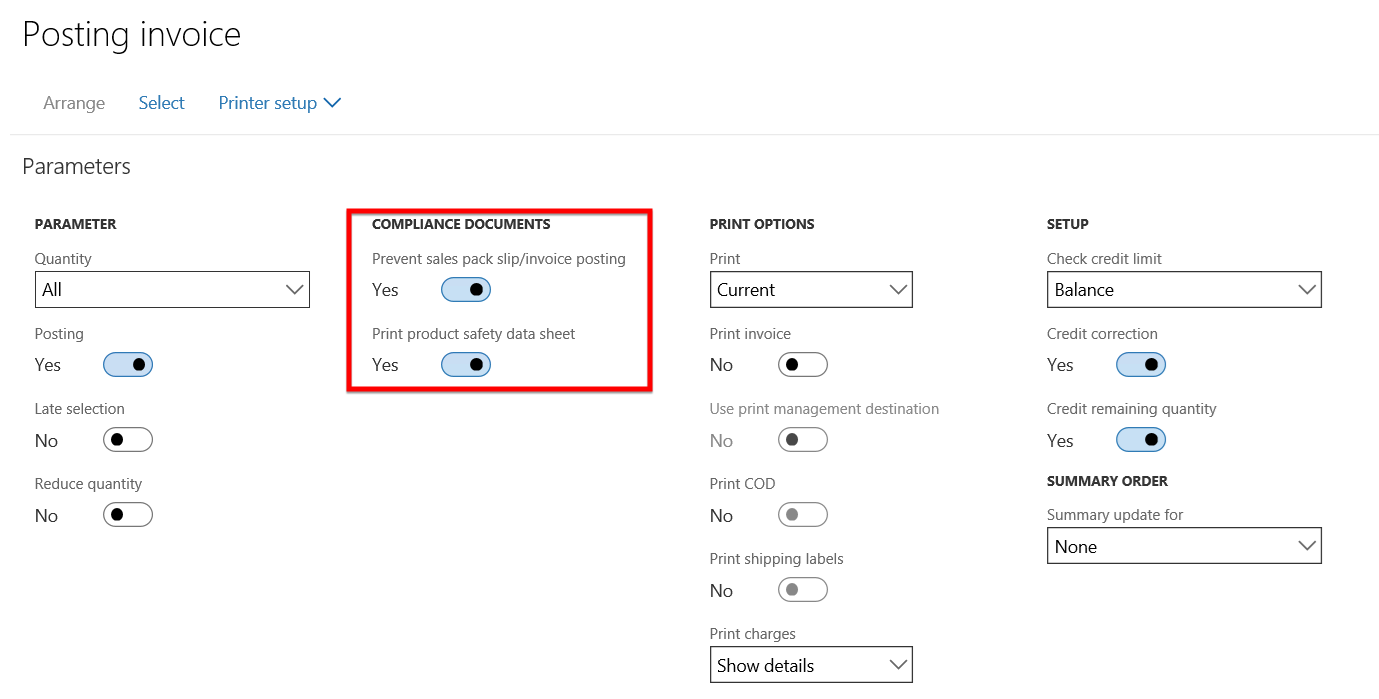
- An error message appears on the Sales order form stating that:
- The latest product safety sheet needs to be delivered to the customer for this product and
- No valid product safety sheet exists for the item on the sales order.
- The Invoice posting has thus been prevented due to the expired product safety data sheet.
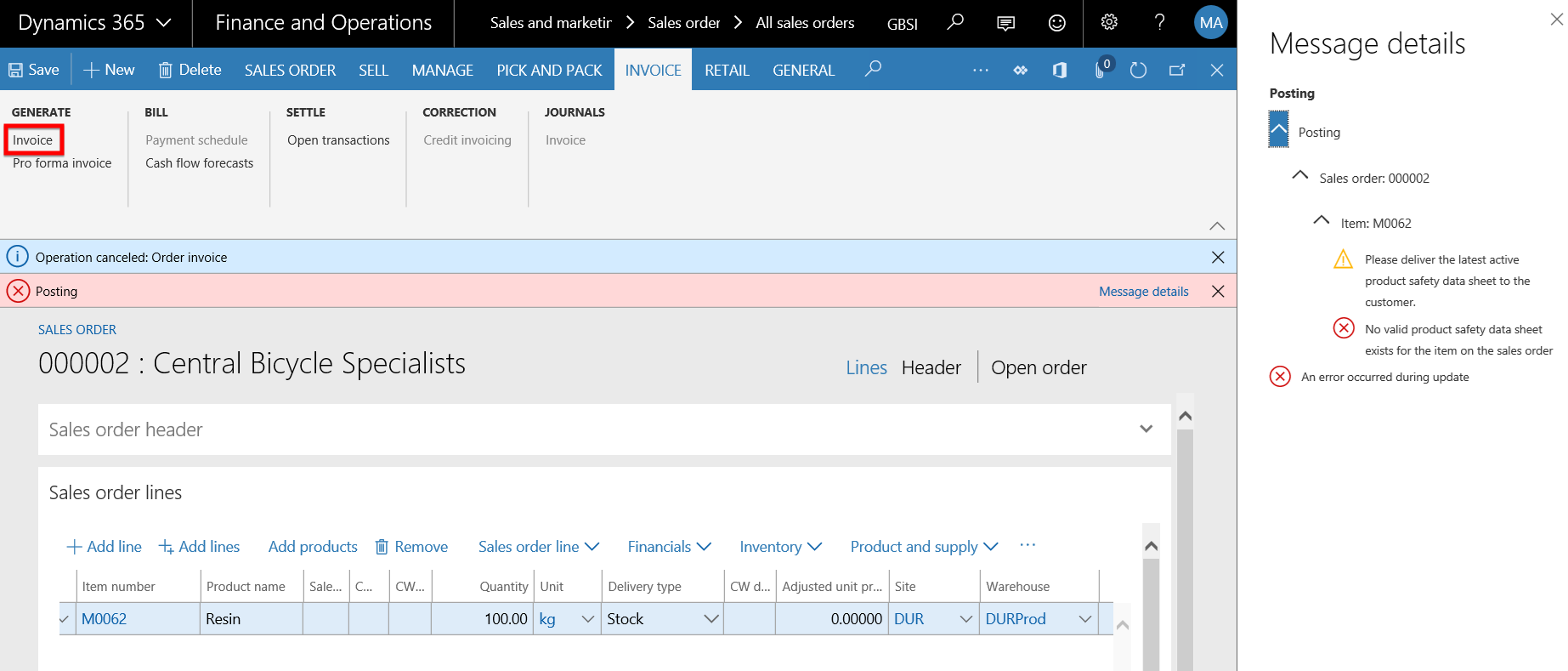
- Follow the same steps as in the Invoice posting step above, but this time set the Prevent sales pack slip/invoice posting field is set to No and post the Invoice.
- The Invoice posts successfully and only the warning message to that the latest product safety sheet needs to be delivered to the customer for this product is displayed to the user.
¶ Scenario 3: Regulated & reported product with expired Product safety sheet purchased
Continuing with the same product M0062 and parameter setup as in Scenario 1, but in this scenario creating a Purchase order would be created to step through the scenario of warnings and Product safety sheet reviews and updates for the Purchase order process. As can be seen in the active Product safety data sheet of product M0062 below, the Expiry date field is a date older than today’s date which means this safety sheet has expired.
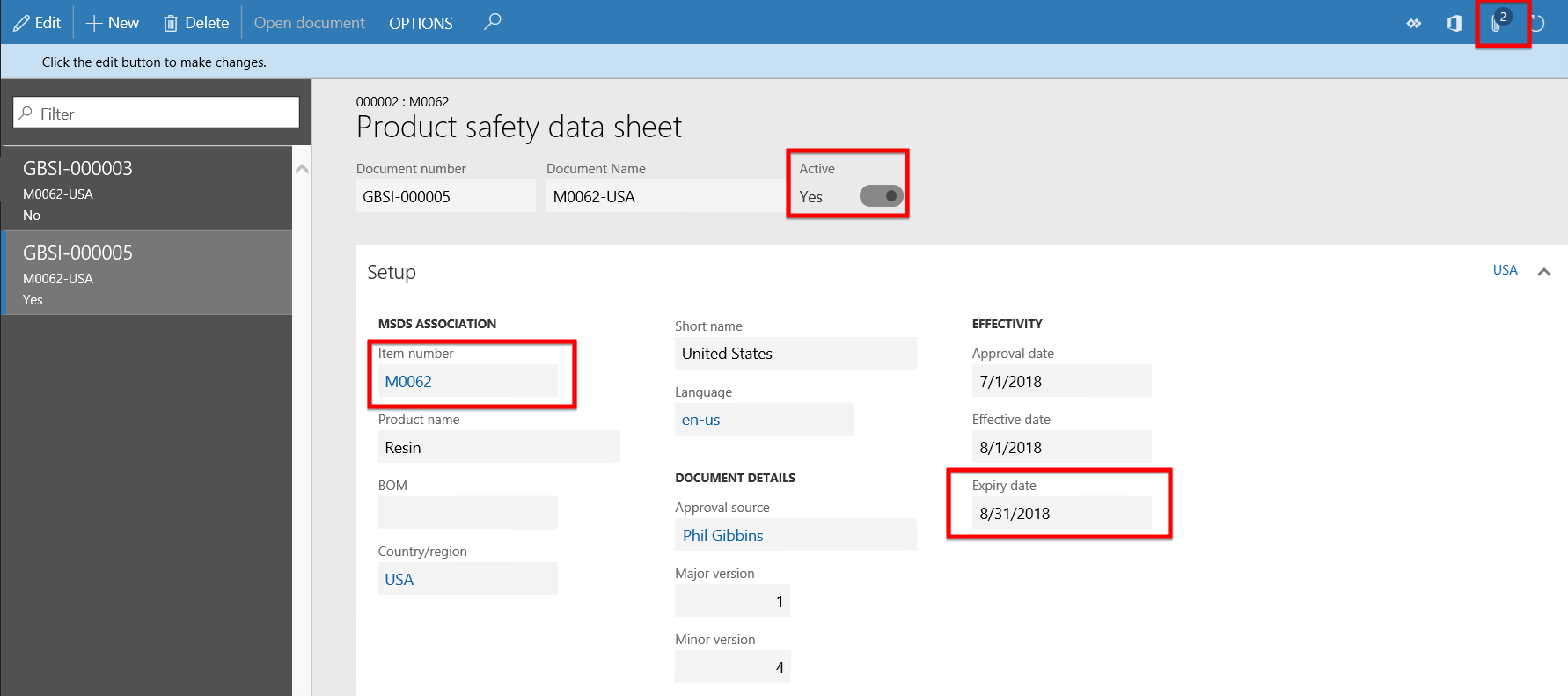
- In this scenario the NY state of USA has been set as an reported regulated product regional list.
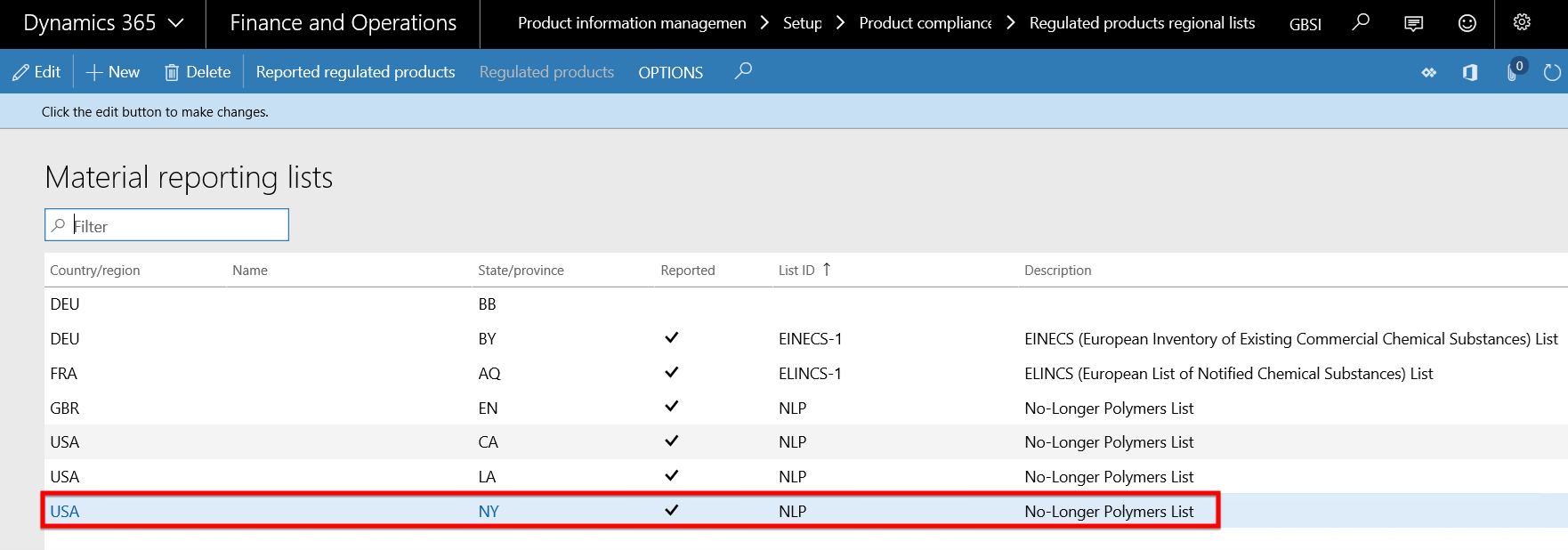
- The M0062 item has been selected and linked to the NY state in USA regulated and reported product regional list record. This means that all purchases (and sales) of item M0062 made to any vendor (or customer) with delivery address in the NY state will be recorded for regulation and reporting purposes.
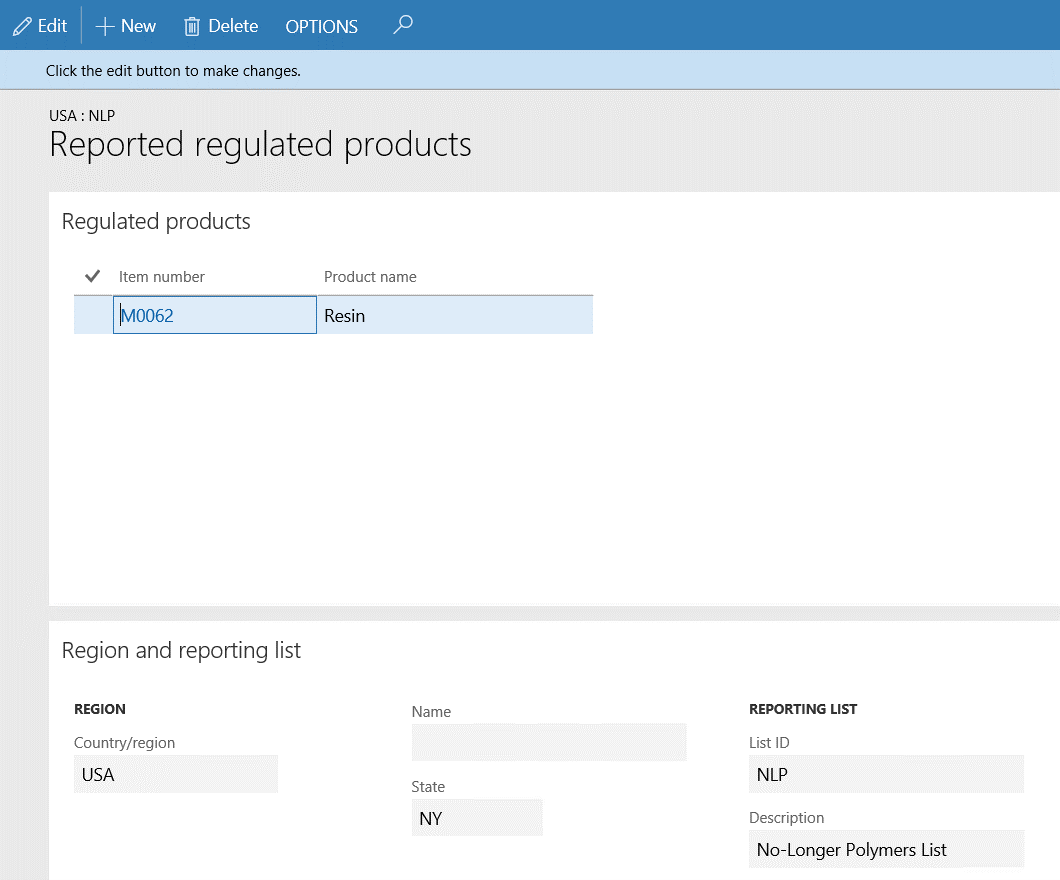
Go to Procurement and sourcing > Purchase orders > All purchase orders
- Create a new Purchase order and select a vendor and address that is in the NY state of USA as can be seen below.
- Now click on the Add line button in the Purchase order lines Fast tab and add item M0062 in the Item number field.
- Also populate the following fields on the Sales order line:
- Quantity
- Unit
- Unit price
- Site (Add through the Sales order line button > Dimension display option of the field does not appear on the Sales order line)
- Warehouse (Add through the Sales order line button > Dimension display option of the field does not appear on the Sales order line)
- Click the Save button
- As the delivery address is regulated and reported for this product and state in the address and the Product safety data sheet product M0062 has expired, the warning message appears warning the user that the Product safety sheet is not on file or has expired. A valid product safety data sheet needs to be requested from this vendor.
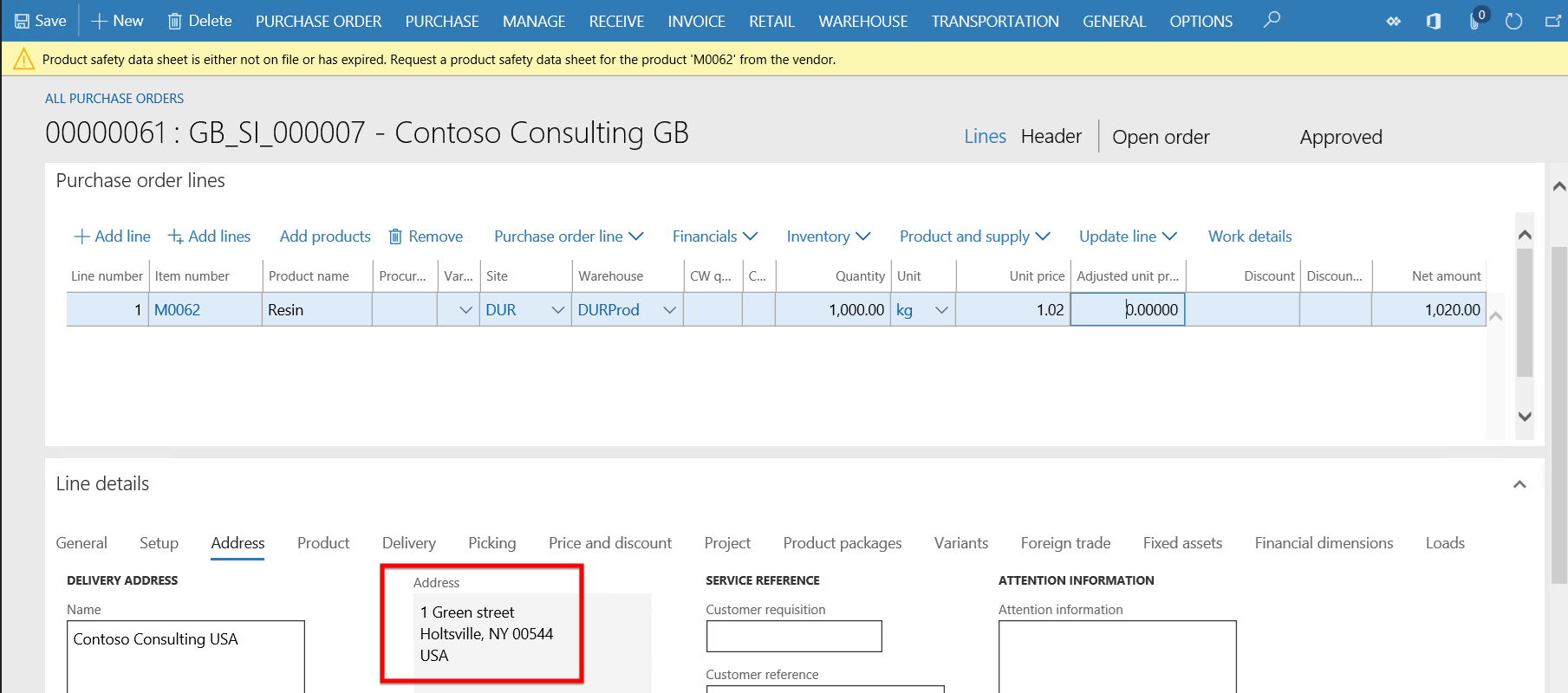
- From the Purchase order line > Inventory button, the Product safety data sheet log can be updated as well as the Safety data sheet accessed and printed if required.
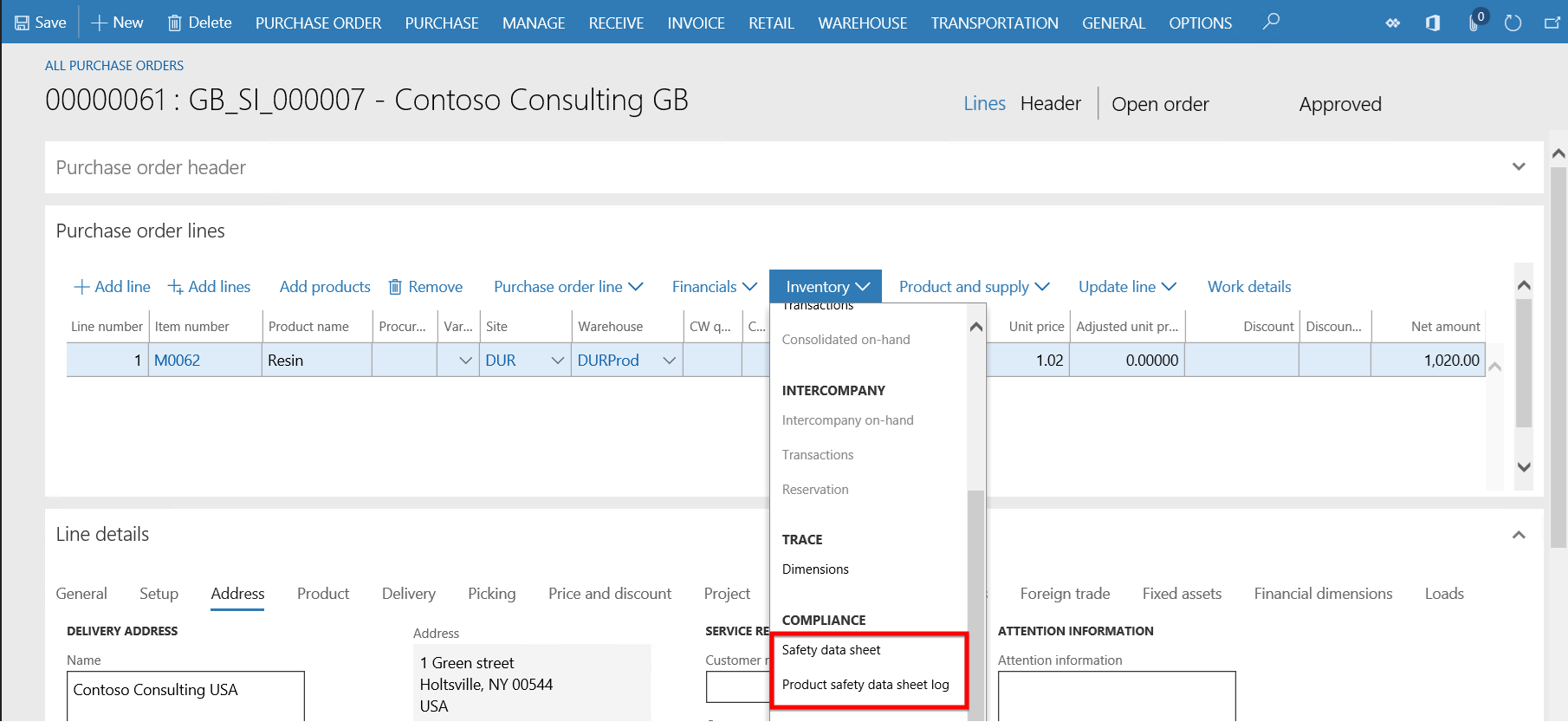
- When the user clicks the Purchase order line > Inventory button > Product safety data sheet log button, the Product safety data sheet log form opens. If a product is regulated or restricted and if a product safety data sheet already exists then it would be an existing record and accessible upon purchase order (or sales order) entry in the Product safety data sheet log form. If a new safety data sheet or a newer version of existing data sheet is received from a vendor then it can be added during purchase order entry. This form keeps a log of all safety data sheets sent or received on each order.
- Add a new line by clicking the New button and populated the relevant field. All these fields are also found on the Product safety data sheet form.

- When the user clicks the Purchase order line > Inventory button > Safety data sheet button, the Product safety data sheet form opens.
- From this form the Product safety data sheet details can be reviewed, updated or opened from Document handling and printed if required.